ALA now funded to enter Phase 1 trial to test new cancer treatment tech on humans…
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 11,376,222 ALA shares and 480,000 ALA options and the Company’s staff own 100,000 ALA shares at the time of publishing this article. The Company has been engaged by ALA to share our commentary on the progress of our Investment in ALA over time.
We all know somebody who has been impacted by cancer.
Current cancer treatments like chemotherapy are destructive to the human body.
Chemotherapy blasts the patient's entire body with radiation to kill a few cancer cells.
It’s a bit like burning down your house to kill a cockroach that's inside.
That's why finding better ways to treat cancer is a +US$120BN market opportunity.
Our Investment ALA is trialling a technology to find, target and attack ONLY cancer cells, leaving the rest of the body healthy and unimpacted.
It’s like being able to find the cockroach in your house and smack it with a newspaper, instead of burning down your whole house to hopefully burn the cockroach too.
ALA’s targeted cancer treatment has already worked in mice with blood cancer.
Not only did the treatment prove to be fast acting, but it also had a higher overall survival rate compared to T cell therapies (another form of cell therapy).
Cell therapy is a new frontier of cancer treatment - it's a type of cancer immunotherapy treatment that uses immune cells that are genetically altered in a lab to enable them to locate and destroy cancer cells more effectively.
As we noted above, ALA’s treatment has worked on mice.
And as of today, ALA is funded to enter trials for their new cancer treatment in humans.
If this trial is successful OR shows early signs of success, it could mean a large commercial deal with a big pharma company.
A commercial deal is what all early stage biotech companies hope to get.
It's more common than you would think in the cell therapy space - given the large potential in the space, big pharma companies are not afraid to throw large sums of cash around.
The most recent major cell therapy transaction was Astrazeneca’s acquisition of Gracell for a total transaction value of US$1.2BN - this was for a Phase 1 stage treatment.
Today, ALA announced it has just raised $12.5M - funds that give it enough runway to begin its own Phase 1 trial...
This was an oversubscribed Placement (at 10c with a 1:1 15c option) with solid support from global institutional investors, including a specialist healthcare/biotechnology fund.
We definitely know it was oversubscribed as we got aggressively scaled back on our own bid.
ALA is capped at $102M at the 10c placement price, now with ~$16.5M in the bank (including the cash it had at Dec 31st 2023).
ALA’s current valuation is just a fraction of some of the larger transactions in the space.
As well as the US$1.2BN deal in December last year, scroll on down to see a full table with all recent deal sizes.
ALA’s oversubscribed capital raise gives a good sense for how much demand there is out there in the market for what the company is trying to achieve with its cell therapy approach.
We’ve been Invested in ALA since February 2022, and over the years have seen ALA progress towards its first clinical trials - knocking down a number of milestones in the process.
The market certainly noticed as well.
After a strong run up in share price - a run up of ~475% that also made it our best performing stock of 2023, ALA has just taken the decision to secure the funds it needed to begin that Phase 1 trial.
The significance of entering a Phase 1 trial is that it may be the trigger for commercial discussions with a large pharmaceutical company...
As we noted above, the most recent major cell therapy transaction was Astrazeneca’s acquisition of Gracell for a total transaction value of US$1.2BN.
Gracell was a T cell therapy company in Phase 1b trials, working on its therapy for multiple myeloma and other blood cancers. (Source)
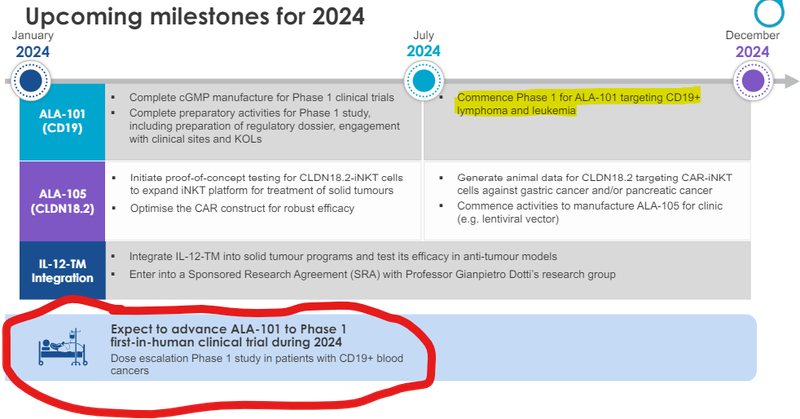
ALA is also working on blood cancers, and is seeking to develop an “off-the-shelf cell therapy” to treat cancer, and will be moving to Phase 1 clinical trials in the second half of 2024.
Cell therapy is a major focus for large pharmaceutical companies right now - as it promises to be a very effective approach to fighting cancer.
So much that many of the transactions happening in the cell therapy space take place either at the preclinical stage or prior to the Phase 2 stage (this is squarely where ALA is right now - pre-clinical, and gearing up for Phase 1 trial):
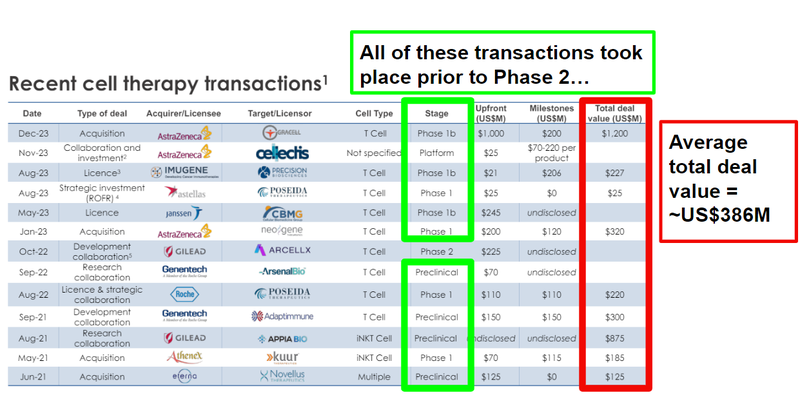
The average total deal value for these cell therapy transactions is ~US$386M.
Following the recently completed raise, ALA will be capped at around ~$102M (at 10c).
This is still a fraction of what some of the acquisition and licensing deals we have seen for cell therapy biotechs.
Cell therapy sits within the field of cancer immunotherapy - a rapidly expanding field within the wider oncology market.
Cancer immunotherapy uses the body’s own defences - for a more in-depth explanation of how cell therapy works, see the end of this article.
For now though, we’re really pleased with the fact this ALA raise was well supported - and what it means for the future of our ALA Investment.
Ultimately this is what the major upside success case looks like for us as ALA Investors:
Our ALA Big Bet:
“ALA achieves a major breakthrough in cancer immunotherapy, and is acquired by a major pharmaceutical company for multiples of our Initial Entry Price”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our ALA Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
What will ALA use these new funds to accomplish?
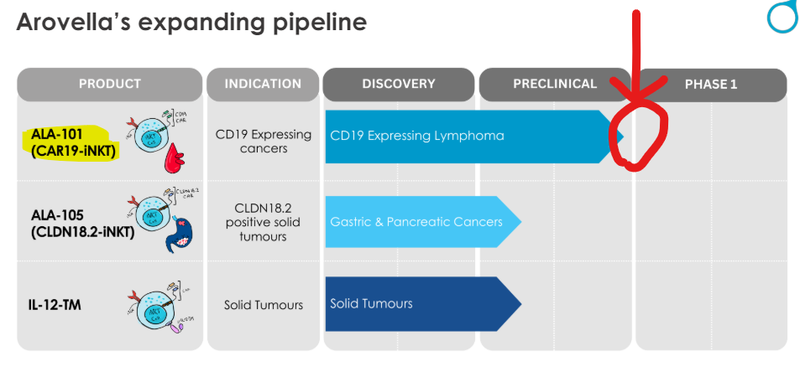
ALA is now fast approaching Phase 1 for its lead product ALA-101, which is its treatment for blood cancers:
A Phase 1 trial is the first phase at which a therapy or treatment is trialled in a human.
A Phase 1 trial “tests the safety, side effects, best dose, and timing of a new treatment. It may also test the best way to give a new treatment (for example, by mouth, infusion into a vein, or injection) and how the treatment affects the body.” (Source)
It may also have some efficacy data as a secondary endpoint. (Source)
An endpoint is, “an event or outcome that can be measured objectively to determine whether the intervention being studied is beneficial”. (Source)
ALA expects to enter its Phase 1 clinical trial by the end of this year:
The blood cancer treatment market is large - worth ~US$70BN in 2023. (Source)
So success in its Phase 1 trial would not only be a powerful proof of concept for ALA’s iNKT cell therapy platform - but potentially immediately valuable from a transaction perspective.
We’ve already seen that ALA-101 works well in mice.
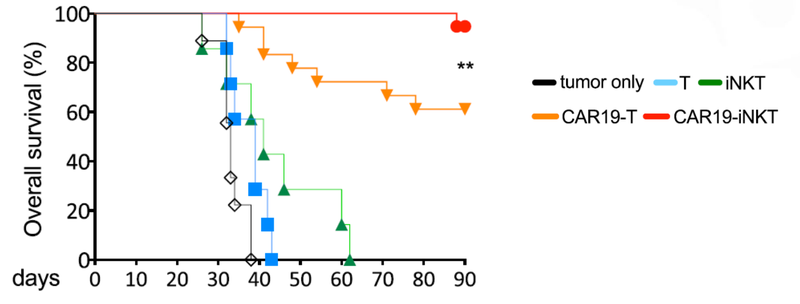
As you can see below, ALA’s therapy on the far right hand side (CAR19-iNKT), killed the cancer faster (removed the coloured blobs) than other similar treatments:
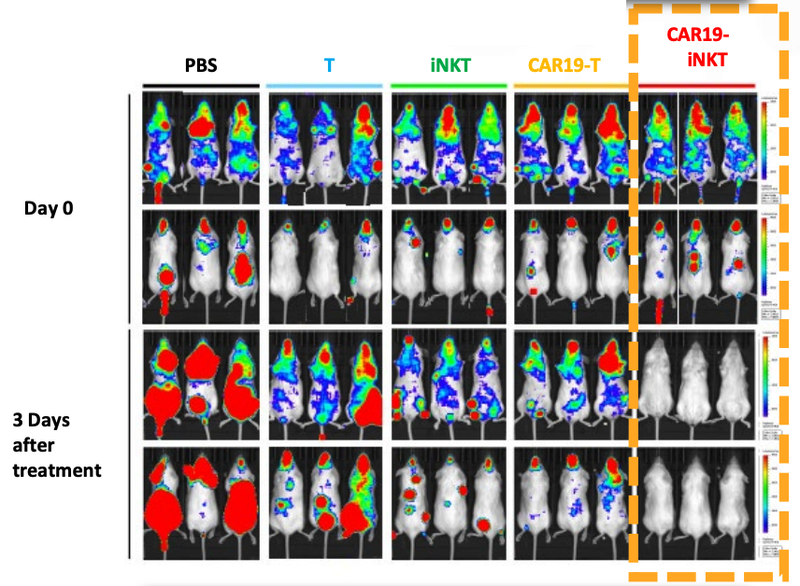
Not only did the treatment prove to be fast acting, but it also had a higher overall survival rate compared to T cell therapies.
This graph shows that at 90 days ALA’s treatment had >90% survival rate, compared to 60% survival rate for T cell therapy:
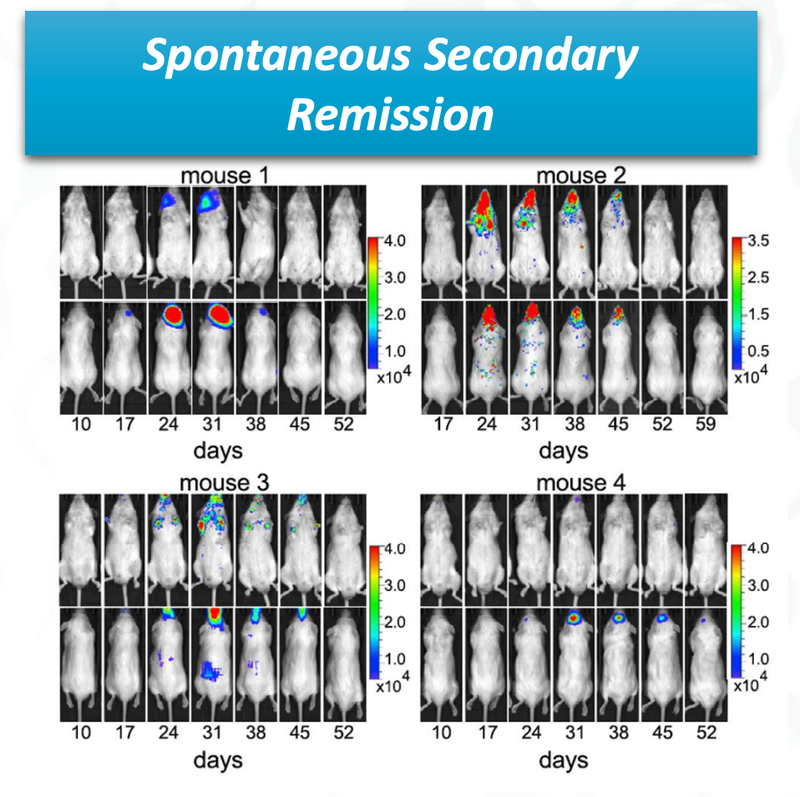
The other really promising thing for ALA, is that there was “spontaneous secondary remission” from a number of mice in the clinical trial, indicating that even if the cancer does come back, the treatment is effective at reducing the secondary onset of cancer cells.
You can see from the image that over time the cancer cells (coloured blobs) come back, but then are soon after destroyed.
This is a significant benefit of the treatment if it can be translated to humans.
Positive test on animals is a good early indicator, but the science will need to be proven out when ALA conducts clinical trials on humans.
That’s what this upcoming Phase 1 trial in humans is all about - and it will be happening this year.
Meanwhile ALA is also advancing ALA-105 for a range of cancers which include:
- Pancreatic cancer
- Gastric cancer
- Gastroesophageal junction cancers
- Potentially even lung cancer
800,000 people per year die of gastric cancer alone and as a result gastric cancer makes up a $10.7BN market. (Source)
Read our commentary: New Deal: ALA can now target lung cancer, pancreatic cancer, gastric cancer
And then it still has its work on solid tumours - driven by the recent acquisition of an “armouring” technology.
Read our commentary on ALA’s armouring technology here: ALA Bolts on New Technology in Cancer Fighting Cell Therapy Platform
Solid tumours make up ~90% of all cancers - so this remains a very promising area for ALA.
What has ALA accomplished since we Invested?
We first Invested in ALA in February 2022 - over two years ago now.
Below are all our articles on ALA since we first Invested.
In addition, all of our Quick Takes on ALA can also be found on our ALA Investment Memo page which gives a quick summary of why we first Invested in ALA.
New Portfolio Addition: Arovella Therapeutics (Feb 2022)
We announced our new portfolio addition Arovella Therapeutics (ASX:ALA) and outlined our Investment Memo for the company.

ALA on the Pathway to Phase 1 Clinical Trials (Apr 2022)
ALA ticked off a key milestone on the pathway to Phase1clinical trials selecting Q-Gen Cell Therapeutics as the manufacturer for its lead cancer treatment.

$23M capped ALA partners with $1.1BN Imugene - Now Targeting 90% of Cancers (Sep 2022)
ALA announced a partnership with $1.1BN capped Immune to develop its technology against solid tumour cancers.

$1.5M annual cost savings allows ALA to focus on what really matters (Oct 2022)
ALA announced that it would phase out its legacy operations to fully focus on the iNKT Cell therapy platform.

We just increased our Investment in ALA - Here’s why (Feb 2023)
We outline why we increased our Investment in ALA, and what we think the future holds for biotech stocks in 2023.

ALA shows it can scale: Pathway towards an “off the shelf” cancer treatment (Apr 2023)
ALA presented new data at a key international conference to industry experts and opinion leaders in the cancer field.

ALA edging closer to clinical trials for “off the shelf” cell therapy cancer treatment (June 2023)
Two new deals announced in the cancer cell therapy space (both north of US$200M), while ALA announced its Share Purchase Plan.

New Deal: ALA can now target lung cancer, pancreatic cancer, gastric cancer (Oct 2023)
ALA announced a deal with “Spartex Group” to licence a technology that gives ALA the ability to target new cancers (pancreatic, gastric, lung).

ALA Bolts on New Technology in Cancer Fighting Cell Therapy Platform (Jan 2024)
Arovella Therapeutics (ASX:ALA) licensed a new technology today that promises to significantly boost the effectiveness of the company’s solid tumour therapy.
🗣️A selection of our favourite ALA Quick Takes:
ALA narrows focus to iNKT cell therapy
Important European patent for iNKT technology secured
ALA gets option for cytokine tech to enhance iNKT platform
Arovella and Imugene therapy kills cancer in a test tube
ALA: Appointment of medtech veteran as Chairman
ALA’s laser-like focus on INkT cell therapy; costs cut
A boost for ALA’s cancer fighting tech?
ALA completes key manufacturing milestone
📺Other videos resources:
Risks
All investments in small cap stocks carry risk.
These are the risks we are most conscious of at the moment for ALA.
Early Stage Biotech Risk
As with many early stage biotechs, a lot can go wrong in developing technology, particularly as ALA has yet to enter a clinical setting.
- The treatment is ineffective
- The treatment is not considered safe for human use
- Patient recruitment is delayed
- Ethics approval is delayed
Manufacturing risk
As ALA has a number of licensed cutting edge technologies, it may encounter difficulties in the manufacturing process.
Combining these technologies may make manufacturing more complex and difficult.
Funding risk
Despite the recently announced $12.5M capital raise, there is always the risk with small caps that more funding is required prior to major catalysts which could cause additional dilution to current holders. However given the new funding injection, this is no longer a short-medium term risk.
Market risk
The market could sell off, or biotechs could sell off as a sector, impacting ALA’s share price regardless of its operational performance.
The market risks for ALA are linked directly to funding risk as capital markets for biotechs remain constrained.
A word on the ALA + Imugene partnership...
We think cell therapy treatment for solid tumours would be the “holy grail” of cancer therapy.
Solid tumours represent 90% of all cancers, and there is no viable CAR-T cell therapy treatment for solid tumours for now, due to a range of technical challenges (Source).
An iNKT cell therapy for solid tumours may be able to overcome some of these challenges.
This is one of ALA’s long term goals - to prove out the viability of its iNKT technology in solid tumours. This will likely become more of a focus for the company after the Phase 1 trials for blood tumours.
In 2022, ALA signed a partnership with ~$768M capped Imugene to evaluate whether combining ALA’s technology and Imugene's technology could be a viable treatment of solid tumours.
This deal was important for the ALA story at the time (when ALA was much smaller) as it had found a credible, bigger partner to develop its technology with.
As part of the partnership, good in vitro (test tube) preclinical trial data was gathered. (Read our Quick Take).
The promising results of the initial Arovella and Imugene preclinical studies were published in 2023, which legitimised ALA’s technology as “one to watch” in this space.
However, last week Imugene decided to end its partnership with ALA. This was before any “in vivo” data could be generated.
We don’t think that this reflects the effectiveness of ALA’s technology’s ability to fight solid tumours as there wasn't even any mice model data produced.
We note that Imugene recently licensed technology from Precision Biosciences for a total deal value of $227M, so the company’s priorities may lie elsewhere.
Beyond that, we simply won’t know the reasons behind the cancellation of the partnership - and we think that this in no way means that ALA’s technology doesn’t work, as it was never even tested in mice models.
ALA is now free to test its treatment on solid tumours with another partner further down the track...
Our ALA Investment Memo
In our ALA Investment Memo you’ll find:
- Key objectives for ALA
- Why we continue to hold ALA
- The key risks to our Investment thesis
- Our Investment plan.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






