Arovella presents new data
Yesterday our preclinical biotech Investment, and one of our big success stories of the year, Arovella Therapeutics (ASX: ALA) announced that it had presented data at a prestigious haematology conference in San Francisco.
We see these conference events as an important part of ALA’s overall efforts to demonstrate the promise, efficacy and technological rigour of its pipeline of cancer fighting therapies.
This time, members of Professor Karadimitris' team at Imperial College London presented a poster at the conference and below is a key figure from ALA’s presentation (our interpretation marked out):
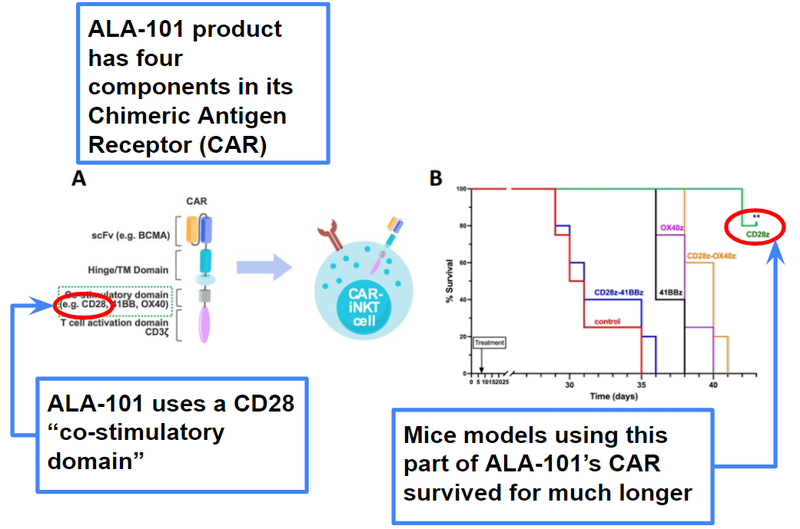
We see this as further evidence of ALA-101’s potential effectiveness in treating multiple myeloma when it progresses to a Phase 1 trial - it also further strengthens the industry and academic credentials of Professor Karadamitris’ work.
Professor Karadimitris is one of the foremost experts at the nexus of iNKT cells and blood cancers and has been publishing iNKT cell research since as early as 2005.
ALA’s iNKT cell therapy platform is a key pillar of ALA’s pipeline of treatments, and is the foundation of ALA-101, the company’s lead candidate for a Phase 1 trial targeted for commencement in 2024.
The other key pillar of ALA’s pipeline is a solid tumour treatment being worked on in partnership with Imugene - which given that solid tumours make up 90% of all cancers, we see as a source of major upside for ALA on any clinical success.
While ALA did put out guidance on 4 December that this particular arm of the pipeline would be slightly delayed due to technical challenges experienced by its contract research provider, yesterday’s data presentation provides us with additional confidence that ALA’s pipeline is progressing well.
Given what’s at stake, we want to see ALA tick all the necessary boxes in a meticulous fashion.
What’s next for ALA?
🔲 Phase 1 clinical trial for ALA-101
ALA-101 is ALA’s most advanced treatment for blood cancers and could be a source of a potential re-rate.
After ALA completes the clinical manufacturing process, it will be all about getting its iNKT cell therapy into Phase 1 clinical trials.
ALA has previously confirmed that it expects phase 1 human trials to occur in 2024.
These are the sub-milestones we are looking for from ALA to make that possible.
- 🔲 Further manufacturing milestones (optimisation, scale up and complete lentiviral vector) -
ALA needs to improve the process, make enough of the therapy to enter a trial and the lentiviral vector can be thought of as the delivery system for the therapy internal to the body.
- 🔲 Finalise clinical trial plan for Phase 1 study -
These are its plans for the Phase 1 study.
- 🔲 Take the therapy to a Phase 1 clinical trial - After all the pre-clinical data is gathered, ALA can choose to progress the therapy into phase 1 clinical trials.
🔲 Phase 1 clinical trial for Solid Tumour Therapy (partnership with Imugene)
At this stage, the partnership is focused on completing proof of concept studies which can then be used to commence phase 1 clinical trials, the process usually follows this route:
- ✅ Prove its most promising treatment works in a test tube - ALA and Imugene have already done this - see our Quick Take on that news here: Arovella and Imugene therapy kills cancer in a test tube
- 🔄 Prove its most promising treatment works in mice - delayed (slightly) - The next step after test tube studies, this will see how the treatment works and if it is safe in a living organism (in vivo - mice studies)






