ALA Bolts on New Technology in Cancer Fighting Cell Therapy Platform
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 13,840,000 ALA shares and the Company’s staff own 575,000 shares at the time of publishing this article. The Company has been engaged by ALA to share our commentary on the progress of our Investment in ALA over time.
Our preclinical biotech Investment Arovella Therapeutics (ASX:ALA) licensed a new technology today that promises to significantly boost the effectiveness of the company’s solid tumour therapy.
ALA was the best performing stock in our portfolio over the 2023 calendar year, and it has hit the ground running in 2024.
We think a big part of ALA’s growing appeal in the market is its continued progress on solid tumours.
The vast majority of cancers are solid tumours - they make up around 90% of all cancers.
Clearly, unlocking the ability to treat these cancers would be a major scientific breakthrough.
ALA’s strategy with licensing technology started with a foundational breakthrough - the iNKT cell therapy platform.
ALA then adds to that platform, by combining other technologies to hopefully develop a powerful cocktail of technology which is effective at killing cancer.
It was only in the last 20 years or so that iNKT cells were looked at for potential cancer fighting properties - and ALA remains to this day one of a small handful of companies advancing the tech.
The ALA difference is that within this field - ALA works with the best researchers and organisations.
By mixing a range of small molecular level breakthroughs from these researchers - we hope the outcome is a major breakthrough.
The tech licensed by ALA today is a “novel armouring cytokine technology” - that allows ALA’s existing therapy to sustain itself in the bloodstream for a longer period of time.
The longer the therapy is in the bloodstream the better it works on at least one type of solid tumour.
New Results Published Today
Today, ALA published some new results pertaining to the technology licensed.
Of the mice treated with a combination of ALA’s existing therapy and the technology licensed today, 75% survived past the 60 day mark.
Of the mice without the combined treatment - none survived past 60 days.
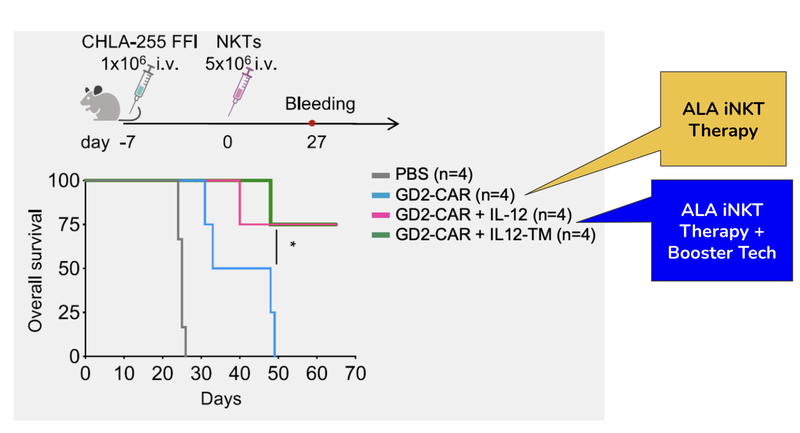
This is very early stage data and ALA is still identifying what technology combinations work best to kill cancer in humans.
However, the data presented today appears to be a strong indication of the armouring technology's effectiveness - enough for ALA to pick up the rights to it.
If ALA can advance a solid tumour therapy to a Phase 1 trial we think this would be a massive leap forward for the company.
And in doing so, ALA could contribute to changing how the world goes about treating cancer.
(And hopefully in the process secure an even more sustained re-rate too).
ALA’s CEO and MD Dr Michael Baker will be presenting a webinar tomorrow to update the market on what this new technology means for ALA:
Click here to register for ALA webinar at 11am AEDT tomorrow
For context, solid tumours have been treated with chemotherapy since the middle of last century - and it wasn’t until 1953 that a first complete cure of a solid tumour was accomplished using chemotherapy.
But chemotherapy remains a blunt, nasty instrument which makes up the backbone of the $327BN cancer treatment market.
Meanwhile the latest big hope in cancer treatments, cell therapy, remains prohibitively expensive and hard to scale.
The vast majority of cell therapy companies focus on something called CAR-T cells - while it has been shown to work - in some ways this has cursed the CAR-T cell therapy industry.
Big pharmaceutical companies are rapidly acquiring CAR-T cell therapy companies for large sums - rewarding oftentimes very small incremental improvements on tech that appears very resistant to cost reductions and widespread use.
At a high level, we think what is holding cancer treatments back at the moment is outdated 70 year old tech (chemo) and a capital allocation problem in the market for new tech (CAR-T cell therapies).
ALA’s is taking a different approach to cell therapy (using something called iNKT cells) - which could be more effective than chemotherapy and solve for the costs and scaling problems found in other cell therapies.
Again, a lot of ALA’s preclinical pipeline relies on the pedigree of the researchers involved.
So it helps that this armouring technology comes from one of the foremost experts in the field of iNKT cells that ALA operates in, within the broader cell therapy field.
Given historical difficulties encountered in treating solid tumours - ALA’s strategy of licensing from the best seems a sound one here.
It’s another string to the bow for ALA - as the company builds out an expanding arsenal of cancer fighting technologies.
Here’s our quick takeaways from today’s ALA announcement
- The data is positive and justifies ALA’s move to exclusively licence it - mice that had a solid tumour and were treated using this tech survived in much greater numbers over a 60 day period.
- The tech comes from a highly esteemed research program - the tech comes from one of the foremost experts in the specialised field that ALA works in.
- The terms of the deal won’t incur any upfront costs to ALA - we think this is another well worked commercial agreement that won’t impact ALA’s cash burn as it approaches a Phase 1 clinical trial this year for blood cancer.
- It de-risks ALA’s preclinical work on solid tumours - animal studies (mice) are usually the precursor to a move into human trials in a Phase 1 study. So this potentially clears a hurdle for the solid tumour side of ALA’s research.
- Potential benefits for Solid Tumour Therapy (partnership with Imugene) - this is important. Today’s announcement indicated that the armouring technology may be able to be combined with the Solid Tumour Therapy that ALA is developing with the $716M capped Imugene.
Since reinventing itself as a cell therapy company, ALA has progressively licensed promising tech four times now.
And with each new technology ALA licences - the overall package of IP under the company's wing (we think) makes it a more attractive target for a major pharmaceutical company.
ALA has made strong progress towards our ultimate goal for our ALA Investment, our Big Bet:
“ALA achieves a major breakthrough in cancer immunotherapy, and is acquired by a major pharmaceutical company for multiples of our Initial Entry Price”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our ALA Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
And as always when ALA makes progress, ALA’s CEO and MD Dr. Michael Baker will be presenting a webinar tomorrow to update the market on what this new technology means for ALA:
Click here to register for ALA webinar at 11am AEDT tomorrow
More on ALA’s new technology
ALA has been performing due diligence on the technology since at least December 2022, when it entered into an option agreement with the developer of the technology.
That was a smart “first dibs” move, but ALA needed to make sure it would work.
This is the person responsible for developing the armouring cytokine technology, Professor Gianpietro Dotti:

Last we checked, a quick search of Professor Dotti’s publications yielded more than 190 works which he has either authored or contributed to - a rough measure of his significant influence in the field of cancer immunotherapy.
And one of his latest works, which is the armouring technology licensed today, was published in the prestigious academic journal Nature Communications:
(Source)
Importantly, Professor Dotti also worked on a program studying the CD19-specific chimeric antigen receptor (CAR) - the very same receptor that ALA is working on in its upcoming slate of preclinical trials which will involve Imugene’s onCARlytics platform.
Previously in June of last year, successful test tube (in vivo) studies triggered a small $15K payment to the University of North Carolina Lineberger Comprehensive Cancer Center where Professor Dotti is based.
And now ALA has thrashed out a deal with UNC Lineberger to licence the tech until 2041.
Here are the terms of the deal as we see them:
- No upfront costs
- A sponsored research agreement and a clinical trial agreement with Professor Dotti’s lab - this is to be negotiated later and we would imagine ALA would contribute in some way to Professor Dotti’s research program. We think this further builds the relationship between the research program and ALA.
- Up to US$10M in total milestone payments, payable on first dosing in a pivotal trial, and on marketing approval for the first two products using this technology - if ALA can achieve these two things we think it is likely to have already re-rated further.
- Future licence payments also include “annual licence maintenance fees” and “low single-digit royalties” associated with commercial sales of the approved products. If ALA gets to this stage we don’t expect these royalties to significantly alter the value of the product - and we think it’s likely ALA would have received a bid from a major pharmaceutical company well in advance of commercial sales.
All up, we see this as a well structured deal for a company at ALA’s stage - it builds a strong relationship with the researcher and doesn’t hamper potential valuation upside in a situation where a major pharmaceutical company may want to acquire ALA for access to IP.
What’s next for ALA?
We think there’s plenty of newsflow to look forward to this year, not least of which is the all important Phase 1 trial for ALA-101, which the company is targeting for commencement in 2024.
Below is a quick summary of all the things we’re looking for from ALA:
🔲 Phase 1 clinical trial for ALA-101
This is the big one.
ALA-101 is ALA’s most advanced treatment for blood cancers and the period leading up to and the start of a Phase 1 clinical trial could be a source of a re-rate.
After ALA completes the clinical manufacturing process, it will be focussed on getting its iNKT cell therapy into Phase 1 clinical trials.
ALA has previously confirmed in its latest video, that it expects Phase 1 human trials to occur in the second half of 2024.
These are the sub-milestones we are looking for from ALA to make that possible:
- 🔲 Further manufacturing milestones (optimisation, scale up and complete lentiviral vector) - ALA needs to improve the process, make enough of the therapy to enter a trial and the lentiviral vector can be thought of as the delivery system for the therapy internal to the body.
- 🔲 Finalise clinical trial plan for Phase 1 study - These are its plans for the Phase 1 study, we expect newsflow around this.
- 🔲 Take the therapy to a Phase 1 clinical trial - After all the pre-clinical data is gathered, ALA can choose to progress the therapy into Phase 1 clinical trials.
🔲 Phase 1 clinical trial for Solid Tumour Therapy (partnership with Imugene)
At this stage, the partnership is focused on completing proof of concept studies which can then be used to commence phase 1 clinical trials, the process usually follows this route:
- ✅ Prove its most promising treatment works in a test tube - ALA and Imugene have already done this - see our Quick Take on that news here: Arovella and Imugene therapy kills cancer in a test tube
- 🔄 Prove its most promising treatment works in mice - delayed (slightly) - The next step after test tube studies, this will see how the treatment works and if it is safe in a living organism (in vivo - mice studies)
🔲Preliminary work on new gastric cancer and pancreatic cancer tech
An October licensing deal with Sparx Group has allowed ALA to expand the types of cancers that it could target.
ALA will be conducting due diligence activities on this technology, with the goal of combining it with its iNKT cell therapy platform:
- 🔄 Manufacturing updates - early work on seeing how the Sparx tech interacts with the iNKT cell therapy platform.
- 🔄 Prove its most promising treatment works in mice - This will see how the treatment works and if it is safe in a living organism (in vivo - mice studies)
Risks
All investments in small cap stocks carry risk.
These are the risks we are most conscious of at the moment for ALA.
Early Stage Biotech Risk
As with many early stage biotechs, a lot can go wrong in developing technology, particularly as ALA has yet to enter a clinical setting.
- The treatment is ineffective
- The treatment is not considered safe for human use
- Patient recruitment is delayed
- Ethics approval is delayed
Manufacturing risk
With this new technology acquired, ALA will need to work out how to manufacture, at scale, its iNKT cell therapy platform together with the new technology.
As new technologies are added, the manufacturing process may become more complex.
We expect that ALA did its due diligence on this front before acquiring the technology, however there is still a risk of manufacturing challenges when ALA looks to produce at scale.
Funding risk
There is always the risk with small caps that more funding is required prior to major catalysts which could cause additional dilution to current holders.
Clinical trials are not cheap and ALA is not generating any material revenue.
ALA had a cash position of $4.7M at 31 December 2023. ALA may need to raise capital in the future to fund clinical trials.
Market risk
The market could sell off, or biotechs could sell off as a sector, impacting ALA’s share price regardless of its operational performance.
The market risks for ALA are linked directly to funding risk as capital markets for biotechs remain constrained.
Our ALA Investment Memo
In our ALA Investment Memo you’ll find:
- Key objectives for ALA
- Why we continue to hold ALA
- The key risks to our Investment thesis
- Our Investment plan.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






