ALA’s cell targeting cancer treatment works in mice - human trials on track with manufacturing scale now achieved
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 8,645,222 ALA shares and 480,000 ALA options and the Company’s staff own 100,000 ALA shares at the time of publishing this article. The Company has been engaged by ALA to share our commentary on the progress of our Investment in ALA over time.
A significant scientific achievement.
And now on track for the highly anticipated Phase 1 clinical in humans trial later this year...
Our biotech Investment, Arovella Therapeutics (ASX:ALA) is developing an iNKT cell therapy platform to fight cancer, and is setting the stage for a Phase 1 clinical trial.
Basically ALA’s treatment can find, identify and kill specific cancer cells, leaving other healthy cells unharmed,
Unlike chemotherapy, which just blasts every cell in the body, even healthy ones.
Cell therapy shapes as a much more targeted approach to fighting cancer than chemo.
ALA has already shown that its treatment works in mice.
ALA’s next step is to show its treatment also works in humans in a Phase 1 clinical trial.
But the key for human trials is to be able to produce a significant volume of the treatment...
This week ALA announced that it has successfully scaled up the manufacturing of the treatment.
Being able to produce a volume of the treatment is also critical IF human trials are successful, to produce enough of the treatment to sell globally.
ALA said - the “process [is] suitable for large-scale and late-phase clinical development” which we take as a big positive.
(but lets hopefully watch ALA pass the human trials first before we start getting excited about global markets...)
With ALA’s first human trials starting this year it should be a very interesting time to follow ALA.
Especially now that ALA has cleared a key hurdle to commence a Phase 1 trial in humans.
Now imagine if ALA can prove their cancer treatment works (and is safe) for use in humans....
Cell therapy is a new frontier of cancer treatment that uses immune cells that are genetically altered in a lab which can locate and destroy cancer cells more effectively.
Instead of blasting and hurting every cell in the body just to hurt the cancer cells (chemo).
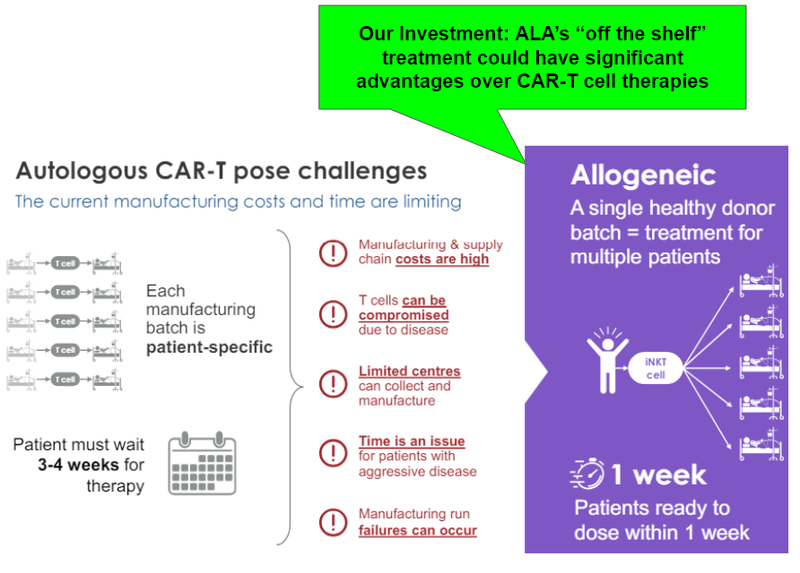
The most common cell therapy is called CAR T cell therapy, and although it is a huge breakthrough in cancer treatment, it does have its drawbacks.
(ALAs is doing iNKT cell therapy which stands as a potential major improvement on CAR T)
Currently CAR T cell therapy costs between US$500,000 and $1,000,000 per patient (source).
The reason for this expense is that each treatment is bespoke to the individual and the technology struggles to scale as a result.
Also, the T-cells used in CAR T cell therapy must be drawn from the individual patient and the treatment must be tailored to an already ailing cancer patient.
This can lead to inconsistent success rates between patients.
ALA has a different approach that uses invariant Natural Killer T (iNKT) cells instead of T-cells from the patient's own body.
(Source)
This makes ALA's therapy an "off-the-shelf" product that does not need to be tailored for each individual patient, potentially making it cheaper and more readily available.
A successful ‘off-the-shelf’ cell therapy technology will have significant commercial value if it is proven to be safe and successfully treats cancer in human trials.
It could mean that a single healthy donor could provide treatments for multiple patients.
And now...
ALA has announced that it has completed the necessary manufacturing milestones (process development) to “scale up” its product for use in human clinical trials.
ALA is currently a “preclinical” stage cancer fighting biotech, and we think that it won’t be too long before the company reaches the “clinical” stage of development.
Basically this means human trials to confirm the treatment is SAFE and EFFECTIVE in humans.
Biotechs that are able to prove this generally appreciate in value, and sometimes draw the interest of large pharmaceutical companies.
Over the past twelve months ALA has been working on “scaling up” the ability to manufacture a consistent and reliable cell therapy treatment that works as intended.
In order to run clinical trials in humans, ALA needs high volumes of the treatment that can be well controlled and reproducible so that each patient gets the same experience.
This is called Good Manufacturing Practice (GMP) - and it is a critical step in any early stage drug development.
(it’s uncommercial if an amazing treatment can be produced once off, but can’t be produced in large, consistent quantities)
ALA has announced it was able to achieve this and develop the ability to make repeatable batches of its treatment with high quality outcomes.
This is no easy feat.
There is a lot of science and testing that goes into making a treatment GMP compliant, and it took ALA nearly 12 months of testing to reach this point.
In the words of ALA CEO Michael Baker “for a cell therapy product, the manufacturing process defines [the] product”.
Now that ALA has completed manufacturing scale up it can begin preparing for a Phase 1 clinical trial in humans.
This involves working with the regulator to complete a trial design, securing ethics approval and commencing the study.
If ALA is able to achieve this by the end of the year we think it will be a big achievement for one of our best performing Investments in the last two years.
The market responded well to ALA as it built out it tech which we knew worked in mice...
now over to ALA to prove that it works in humans...
ASX:ALA
Arovella Therapeutics
ALA is developing an “off the shelf” cell therapy platform to treat cancer.
Its product is a modified iNKT cell that has a CAR-19 to help target and kill cancer cells.
iNKT cells are a natural part of the body’s immune defence system (the bow), whereas the CAR-19 is a receptor that can be attached to an iNKT to target and kill cancer cells (the arrow).
Together they make ALA’s cancer killing product:
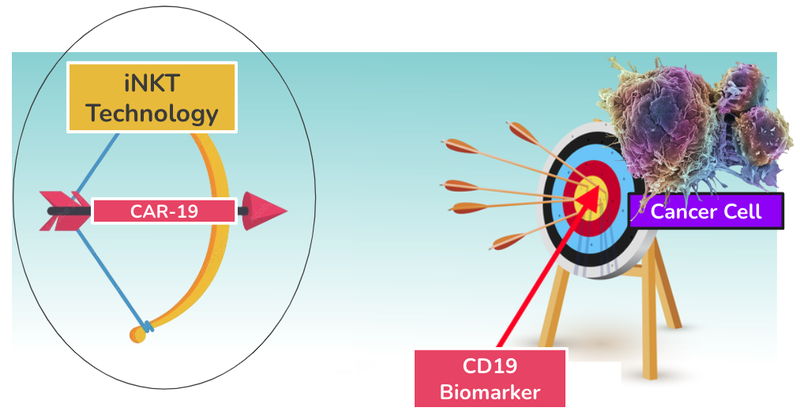
ALA has been able to combine these two components that don’t occur naturally together to create a proprietary treatment for cancer.
Think of this as ALA’s prototype.
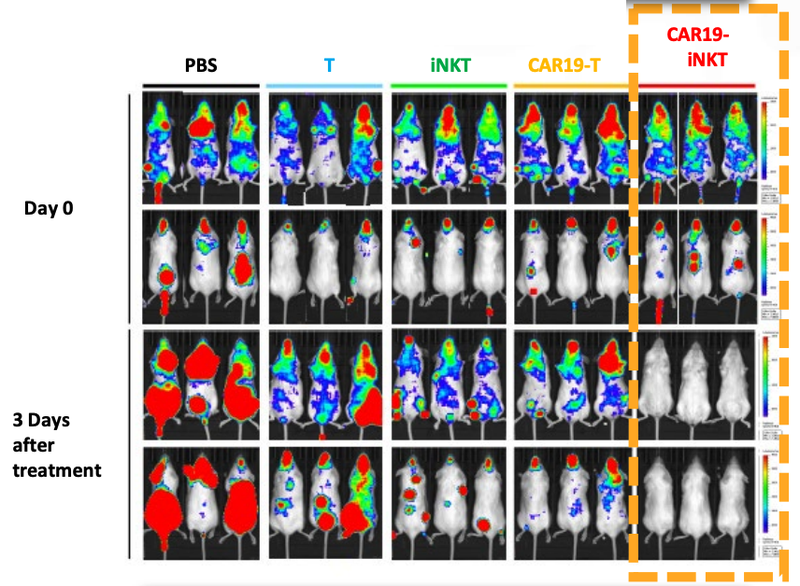
The company has tested this ‘prototype’ on mice with extremely positive results.
As you can see below, ALA’s therapy on the far right hand side (CAR19-iNKT), killed the cancer faster (removed the coloured blobs) than other similar treatments:
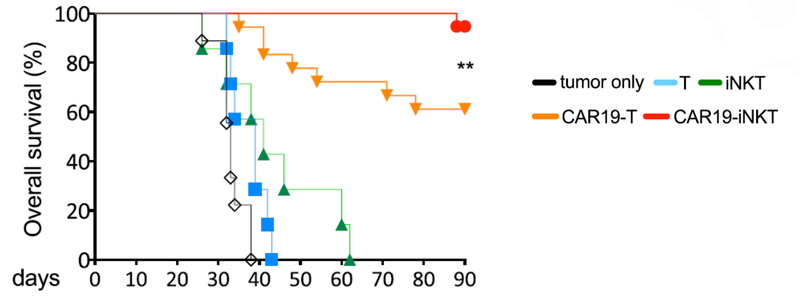
Also, ALA’s treatment had a higher overall survival rate compared to T cell therapies.
This graph shows that at 90 days ALA’s treatment had >90% survival rate, compared to 60% survival rate for T cell therapy:
The final promising data from ALA’s preclinical trial is that there was “spontaneous secondary remission” from a number of mice.
You can see from the image that over time the cancer cells (coloured blobs) come back, but then are soon after destroyed.
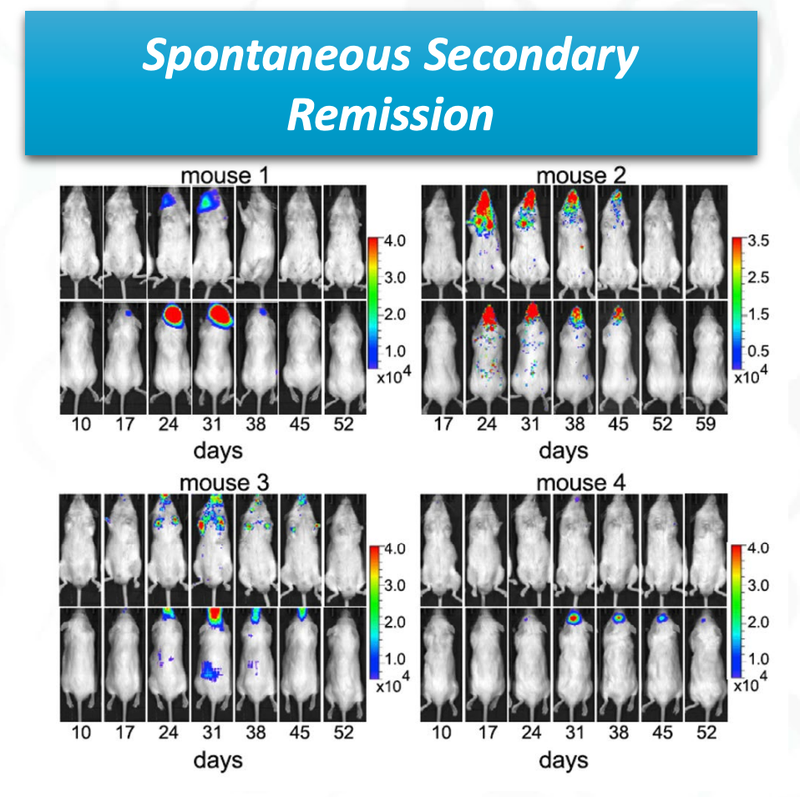
The next stage for ALA is to see if it can repeat these promising developments in humans, in what is called a clinical trial.
In order to run clinical trials in humans, ALA needs more of its cancer killing product.
Being able to reproduce results is a foundational element of science, and to do this, ALA has been working on “scaling up” manufacturing and production to try and get a consistent product that is of high quality.
This week ALA announced that it had achieved this and was able to produce “a high yield of CAR-19 positive iNKT cells with very high purity.”
This is no small feat and stands as a major de-risking event for its cell therapy technology.
Importantly, the final product characteristics are consistent with product safety and quality requirements by the FDA - which is important as ALA moves closer towards a phase 1 clinical trial.
Ultimately, we want to see if ALA can achieve a major breakthrough in cancer immunotherapy and prove its technology through the clinical pathway.
This brings us to our Big Bet for ALA:
Our ALA Big Bet
“ALA achieves a major breakthrough in cancer immunotherapy, and is acquired by a major pharmaceutical company for multiples of our Initial Entry Price”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our ALA Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
What cancers is ALA’s “off the shelf” treatment trying to treat?
ALA’s first Phase 1 trial is targeting blood cancer, which is still a large market worth roughly US$75BN this year (source).
However, the company has also licenced, and is working on, a product that targets other forms of cancer including:
- Pancreatic cancer
- Gastric cancer
- Gastroesophageal junction cancers
- Potentially even lung cancer
This is in addition to a treatment for solid tumours which ALA is still working on - solid tumours make up 90% of all cancers.
From a scale up perspective, ALA has said that “the manufacturing process can be applied to all of Arovella’s future CAR-iNKT cell products”.
Which means that future cell therapy treatments that ALA may look to develop may have a reduced lead time to transition from preclinical to clinical trials.
Why “process development” and scale up is important for ALA...
ALA has developed a modular, semi-automated process that is suitable for large-scale and late-phase clinical development.
This is particularly important for ALA’s “off the shelf” cell therapy where scalability is the name of the game.
(As opposed to the bespoke, expensive “1 patient at a time” CAR-T cell therapies which have attracted billions of dollars in biotech money already).
Remember, the goal for ALA is to treat a large number of patients all at the same time, driving down costs.
We imagine there are a lot of advanced techniques used in this process, and a range of high end equipment.
Luckily though, ALA notes that the process uses well-known automated cell therapy equipment, meaning that a range of jurisdictions can perform the process as well.
Agan, being able to reproduce results is a foundational element of science, which is why ALA needs more of its product at a high quality to run human trials.
Ultimately, this manufacturing scale up was a major hurdle that ALA successfully cleared and a testament to the strong scientific capability of the ALA team.
Next, we are looking forward to ALA preparing for a Phase 1 clinical trial, to see if it can repeat the preclinical success of its product.
What’s next for ALA?
🔲 Commence Phase 1 clinical trial for ALA-101
This is the big one.
ALA has previously confirmed that Phase 1 human trials will occur in the second half of 2024.
ALA-101 is ALA’s most advanced treatment for blood cancers and the period leading up to and the start of a Phase 1 clinical trial could be a source of a re-rate.
These are the sub-milestones that ALA will likely need to achieve in anticipation of the Phase 1 clinical trial:
Milestones
✅ Complete process development and scale up - Completed this week
🔄 Progressing to engineering and GMP batches to produce material for trial
🔲 Announce clinical trial plan
🔲 Secure ethics approval
🔲 Commence clinical trial
🔲 Preliminary work on new gastric cancer and pancreatic cancer tech
In October last year ALA signed a licensing deal with Sparx Group that allowed the company to expand the types of cancers that it could target.
ALA will be conducting due diligence activities on this technology, with the goal of combining it with its iNKT cell therapy platform:
🔄 Manufacturing updates - early work on seeing how the Sparx tech interacts with the iNKT cell therapy platform.
🔄 Prove its most promising treatment works in mice - This will see how the treatment works and if it is safe in a living organism (in vivo - mice studies)
Risks
Early Stage Biotech Risk
As with many early stage biotechs, a lot can go wrong in developing technology. As ALA moves towards the clinical trial phase these risks will be more pronounced:
- The treatment is ineffective
- The treatment is not considered safe for human use
- Patient recruitment is delayed
- Ethics approval is delayed
Funding risk
Despite the recently announced $12.5M capital raise, there is always the risk with small caps that more funding is required prior to major catalysts which could cause additional dilution to current holders.
However given the new funding injection, this is no longer a short-medium term risk.
Market risk
The market could sell off, or biotechs could sell off as a sector, impacting ALA’s share price regardless of its operational performance.
The market risks for ALA are linked directly to funding risk as capital markets for biotechs remain constrained.
Our ALA Investment Memo
In our ALA Investment Memo you’ll find:
- Key objectives for ALA
- Why we continue to hold ALA
- The key risks to our Investment thesis
- Our Investment plan.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






