A boost for ALA’s cancer fighting tech?
Today our preclinical biotech Investment, Arovella Therapeutics (ASX: ALA) has revealed that new data from experiments indicate that its iNKT platform technology can be enhanced by another complementary technology.
We hope this could improve the effectiveness of its cancer fighting technology which has shown promise in mice studies, even demonstrating spontaneous secondary remission in these models (Slide 17).
This is specifically the cytokine technology it secured an exclusive option agreement on in December 2022.
The simple version of this development is that we see this cytokine technology as essentially a “booster” for ALA’s tech.
I.e when ALA first secured this agreement, the company indicated that the tech could increase the ability of iNKT cells to persist for longer - so they don’t get cycled out of the body as quickly - boosting their effectiveness.
This is high end tech and comes via University of North Carolina Lineberger Comprehensive Cancer Center, and the highly esteemed Professor Gianpietro Dotti who has over 190 works which he has either authored or contributed to in the field of cancer immunotherapy.
The data has come in looking positive, so now we will be looking to see if ALA enters into a definitive licence agreement to formalise ALA’s IP position with this tech.
ALA has developed a strong track record of licensing promising cancer fighting tech from elite institutions - with its iNKT cell therapy platform being sourced from Imperial College London and its DK-1 CAR The University of Texas MD Anderson Cancer Center.
We think this approach is the right one, and ALA is doing a good job at differentiating itself from other iNKT cell companies, of which there are just a handful.
We note that one of these iNKT cell focussed companies recently went into administration after some corporate difficulties.
ALA recently raised $4.1M via a placement and is looking to raise a further $1M via a Share Purchase Plan (SPP), both at 4.5 cents a share - ALA is currently trading at 4.6 cents.
Below are the relevant dates for the completion of the ALA SPP:
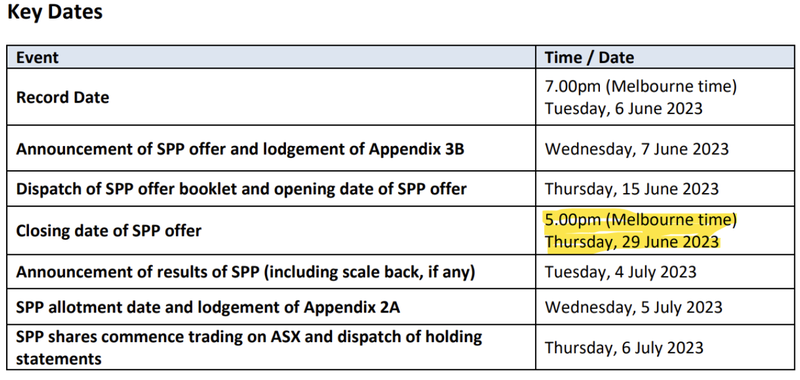
The offer closes 29 June which is Thursday and just 3 days away.
We Invested in the 2c raise in January 2023.
We’ve got high hopes that ALA can deliver on important milestones and key objectives.
What’s next for ALA?
Read more in our latest ALA note.
NEW: Enter into definitive licensing agreement for “booster tech” 🔄
See above.
Phase 1 clinical trial for ALA-101 🔲
After ALA completes the clinical manufacturing process, it will be all about getting its iNKT cell therapy into phase 1 clinical trials.
ALA has previously confirmed that it expects phase 1 human trials to occur in 2024.
Phase 1 clinical trial for Solid Tumour Therapy (partnership with Imugene) 🔲
At this stage, the partnership is focused on completing proof of concept studies which can then be used to commence phase 1 clinical trials, the process usually follows this route:
- Prove its most promising treatment works in a test tube ✅ - ALA and Imugene have already done this - see our Quick Take on that news here: Arovella and Imugene therapy kills cancer in a test tube
- Prove its most promising treatment works in mice 🔄 - The next step after test tube studies, this will see how the treatment works and if it is safe in a living organism (In vitro - mice - studies)
- Take the therapy to a Phase 1 clinical trial 🔲 - After all the pre-clinical data is gathered, ALA and Imugene can choose to progress the therapy into phase 1 clinical trials.
Bonus: Advance treatment #2 (ALA 104, DKK1-CAR-iNKT) 🔄
While Solid Tumour Therapy (with Imugene) is the big shiny target, we’re also looking for ALA to advance this treatment for a range of less prominent, but still important, cancers.






