Why biotechs could be about to go for a run again…
Published 08-JUN-2024 10:00 A.M.
|
9 minute read
What Happened?
- Commentary: Deja vu? We've seen this before. Biotechs taking centre stage again? This guy thinks so.
- Quick Takes: PFE, NTI, 88E,GUE, CND, BPM, GTR
- This week in our Portfolios: GAL, TEE, ALA
Is that a whiff of late 2020 we smell?
People talking about a silver squeeze again?
GameStop back on the menu?
...and it started with Canada?
This week the Canadian central bank cut rates,
...and the European Central Bank followed shortly after.
And while we may have to wait a bit longer in Australia, the central bankers are now starting to make moves.
We have already seen small caps start to respond to positive news and stay higher.
And we think the same could start to happen for most companies that are pre-revenue...
Think tech, biotechs and junior explorers in the small cap end of the ASX.
Three sectors we think will benefit the most is:
- Small cap resources companies - The ASX is home to some of the worlds biggest mining companies and is in our opinion the number one exchange to find solid resources Investments.
- Small cap biotechs - Australia is a good place for early stage biotechs to benefit from R&D tax incentive schemes and skilled research personnel.
- Small cap tech companies - Tech stocks are typically cash flow negative while they are in a high growth phase. Easier access to capital will mean they can invest in growth and get to where they want to be quicker.
We spoke about small cap resources companies last weekend: Small Cap market is looking good... and are share price re-rates back?
Biotechs have been a good sector for us over the last 18 months, while the rest of the market was terrible.
Our 2022 Biotech Pick of the Year Dimerix (ASX:DXB) hit new multi year highs on Friday and the run looks like it isn’t stopping.
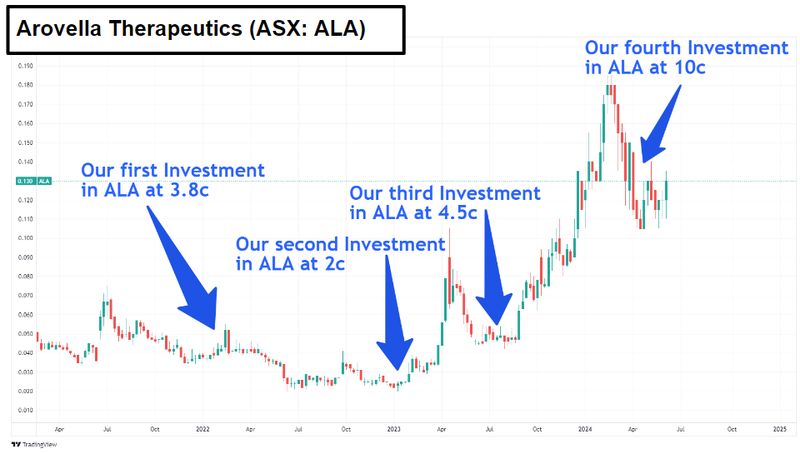
Another of our biotech Investments Arovella Therapeutics (ASX:ALA) looks to be on the move back up now too.
This weekend we look at why biotechs could be about to go for a run again...
Biotech boom?
We came across an interesting article in the AFR this week...
It was an interview with fund manager Rory Hunter from SG Hiscock - a Melbourne based medical technology fund with $4.2BN under management.
In the interview a lot was discussed about the state of play in the biotech space.
The general gist of the article was that SG Hiscock thinks the ASX could enter a boom period for biotech stocks.

(Source - AFR)
In the article Rory mentioned two companies that we have Investments in:
First mention was Dimerix (ASX:DXB)
Rory picked it as one of the companies he is watching that could replicate the success of Neuren Pharmaceuticals in the rare diseases space.

For some context, Neuren’s share price re-rated by over 1,300% in three years off the back of FDA approval and subsequent commercialisation of a treatment for a rare disease - Rett Syndrome.
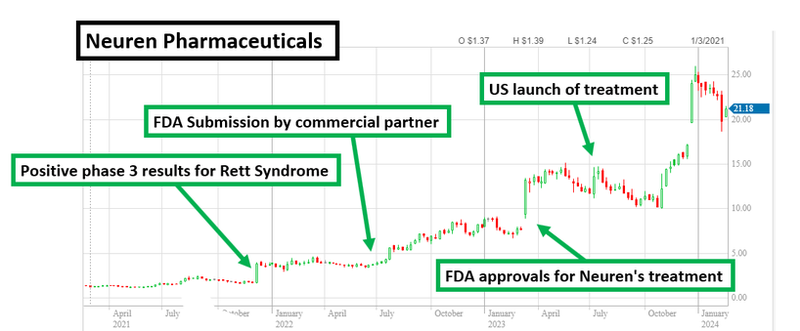
We have another company that is working on a treatment for Rett Syndrome - the same disease Neuren targeted - Neurotech International (ASX: NTI)...
Read our latest NTI note here: NTI’s Phase I/II Rett Syndrome clinical trial results improve upon $2.4BN capped Neuren’s results...
Rory reckons DXB is looking to do something similar to Nueren too.
DXB is about to finish a Phase 3 on its treatment for a rare kidney disease called FSGS.
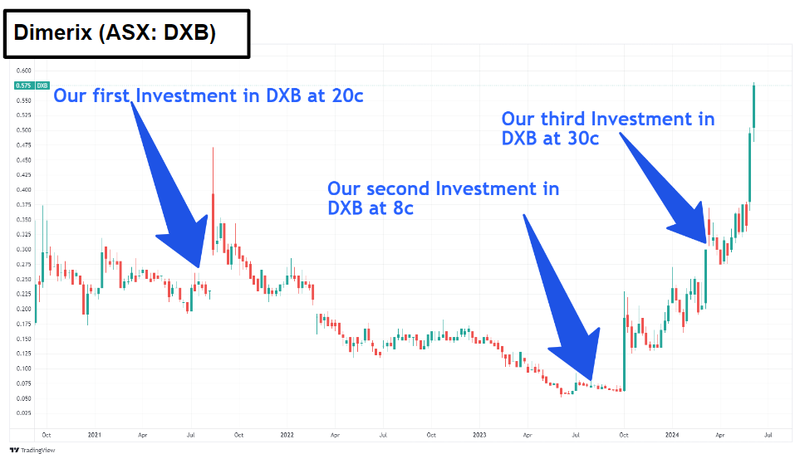
To date, DXB has signed licensing deals worth up to ~$340M in milestone payments and ~$11.5M in upfront payments.
A licensing deal is good for early stage biotechs like DXB because in most circumstances it gives the small biotech company:
- Funding to complete its trials without diluting shareholders
- Certainty around commercialisation, and
- Product validation from a market perspective for how big pharma values a treatment
The catch is normally that most of the funds hit the small companies bank account if the treatments end up working and actually get delivered to market...
So far, DXB’s deals cover Europe, UK, Australia, NZ, Canada and parts of the Middle East.
At the moment DXB is yet to sign a deal for the two biggest markets in the world...
USA and China.
That’s where we are hoping the next big DXB catalyst comes from.
We noticed on DXB’s Linkedin that the company recently attended the BIO 2024 conference which is held in San Diego in the US.
Interestingly, the conference is said to be the “largest pharma partnering conference in the world”...
We can only assume that DXB is working hard to get some sort of deal done in the US too...
It's been a strong run up in the last year, but we think the big re-rate could happen if DXB successfully delivers two specific catalysts:
- Complete Part 2 of the Phase 3 trial on a nasty rare kidney disease called FSGS - at which point DXB could apply for conditional marketing approval which would allow for commercialisation of the treatment.
- US/China licensing deal - this could happen at any time if an interested party comes to the table and signs a deal for DXB’s treatment. DXB CEO Nina Webster is well versed in signing licensing deals in previous roles (and already has two deals tucked away for DXB at the moment). We’re hoping a further licensing deal comes in a major market like the US or China.
Second mention was Arovella Therapeutics (ASX:ALA)
In the AFR biotech fund manager interview, Rory also touched on another one of our Investments - ALA.
When asked “Which stocks in the fund could be M&A targets?” Rory made mention of ALA pointing at the company’s “exciting and compelling early stage data”.

We tend to agree with Rory...
ALA is developing an off the shelf iNKT cell therapy platform to fight cancer.
At a very high level the treatment would find, identify and kill specific cancer cells without any “collateral damage” to the patients' healthy cells.
ALA has already shown that its treatment works in mice and this week made a big breakthrough in terms of manufacturing its treatment.
ALA announced that it has successfully scaled up manufacturing of the treatment to the point where it can start producing enough to run Phase 1 clinical trials in humans.
Those clinical trials are expected to start by the end of the year.
Coming back to what Rory was talking about (M&A target potential)...
ALA is currently capped at $136M and after its recent capital raise had ~$15.31M cash in the bank.
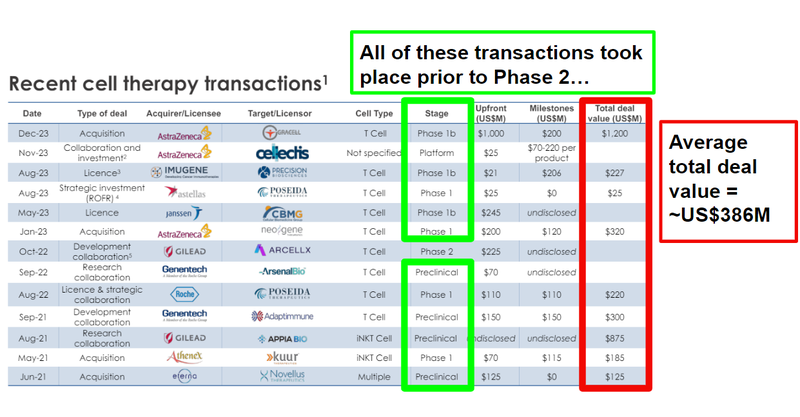
But ALA’s current valuation is just a fraction of some of the larger transactions in the space.
In December last year AstraZeneca did a deal with T-Cell focused Gracell worth up to US$1.2BN (source).
And over the last few years there have been multiple other multi hundred million dollar deals done in the cell therapy space.
The average total deal value for these cell therapy transactions is ~US$386M.
With ALA preparing to go into Phase 1 clinical trials we think the company is approaching what could be an inflection point for the business...
Either:
- ALA goes into clinical trials and delivers a good/bad result which would re-rate the share price higher or lower depending on the outcome
OR
- A bigger pharma company looking for exposure to the potential upside of that phase 1 clinical trial comes in and takes out ALA.
It's still very early days but we are looking forward to seeing what ALA does over the coming months...
We are looking to make new biotech Investments
In a weekender we wrote in September last year we mentioned why we thought Biotech stocks were looking like an interesting investment opportunity:
(Source - Weekender, September 2023)
In that article we also mentioned why we were looking to make more Biotech Investments:

(Source - Weekender, September 2023)
Between September and now we added a few biotech Investments to our portfolio:
- Neurotech International (ASX: NTI) on the 18th of Septermber 2023 - NTI is developing treatments for disorders such as Autism Spectrum Disorder, Rett Syndrome, PANDAS/PAN and Cerebral Palsy, check out our NTI Investment Memo here.
- Emyria (ASX: EMD) on the 4th of September 2023 - EMD is developing psychedelic-assisted therapies using MDMA, ketamine and psilocybin to treat difficult mental health problems, check out our EMD Investment Memo here.
- TrivarX (ASX: TRI) on the 2nd of May 2024 - TRI is developing an AI-driven technology that screens and detects mental health conditions. Check out our TRI Investment Memo here.
Luckily for us, biotechs went for a run a few months after we made the bulk of our Investments...
At their respective peaks, NTI was up ~190% from our Initial Entry Price, and ALA was up ~387% from our Initial Entry Price.
We think that after taking a short breather, biotechs could be about to go on another run.
Especially with market sentiment improving and rate cuts starting to factor into market structure.
We are running the ruler over a few and hope to finalise a few Investments over the coming weeks.
If you have any biotech stocks you want us to take a look at reply to this email with a ticker code.
How we Invest in early stage biotech companies:

(Source - 🎓How we invest in early stage biotech companies)
What we wrote about this week 🧬 🦉 🏹
Arovella Therapeutics (ASX:ALA)
ALA has cleared a key hurdle to commence a Phase 1 trial in humans - this week ALA announced that it has successfully scaled up the manufacturing of the treatment.
ALA said - the “process [is] suitable for large-scale and late-phase clinical development” which we take as a big positive.
Read more: 🧬 ALA’s cell targeting cancer treatment works in mice - human trials on track with manufacturing scale now achieved
Top End Energy (ASX: TEE)
This week TEE was granted the first NEW gas permit in the Northern Territory in almost 10 years...
For almost two years TEE has been working on getting its ground permitted around the Beetaloo Basin in the NT - permitting has been the major hurdle for TEE to start working up its projects.
With one of the priority permits granted, TEE can now work up its project through to what we hope is a high impact drilling event in the months/years to come.
Read more: 🛢️ TEE granted first new NT gas permit in 10 years as Australian prioritises gas
Galileo Mining (ASX: GAL)
On Monday GAL came out of a trading halt having signed a lithium JV agreement with $14BN Mineral Resources (MinRes).
The JV agreement see’s GAL receive $7.5M ($5M upfront and $2.5M in 12 months time) for a 30% interest in the lithium rights at GAL’s Norseman assets.
The deal excludes all of GAL’s existing nickel-PGE discovery where GAL recently finished a round of drilling and is expecting assay results from.
Read more: ⛏️ $14BN MinRes to pay GAL $7.5M for 30% of its Lithium Rights
Quick Takes 🗣️
PFE smashes through 20K acre mark for lithium acreage
NTI’s PAN/PANDAS clinical trial results get even better
88E releases internal resource estimate for Project Leonis
GUE releases uranium intercepts
Evidence builds for CND’s big offshore gas play in Peru
BPM to drill next to $1.7BN Capricorn Metals in July
GTR appoints Ex Cameco Australia director
Bite sized summaries of the latest mainstream news in battery metals, biotechs, uranium etc: The Future Money: https://future-money.co/
Macro News - What we are reading 📰
Gold:
You Can Now Buy Gold Bars From Vending Machines in South Korea (Bloomberg)
Iron ore:
Iron ore to plunge below $US100 into a bear market: Citi (AFR)
Have a great weekend,
Next Investors
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

