NTI’s PAN/PANDAS clinical trial results get even better
Even after successfully meeting a primary endpoint - the good results keep rolling in…
Positive news today from our biotech Investment, Neurotech International (ASX: NTI) which is doing clinical trials on a treatment for a range of neuropsychiatric disorders.
Today’s results are from a rare paediatric condition called PANDAS/PANS.
Key takeaways:
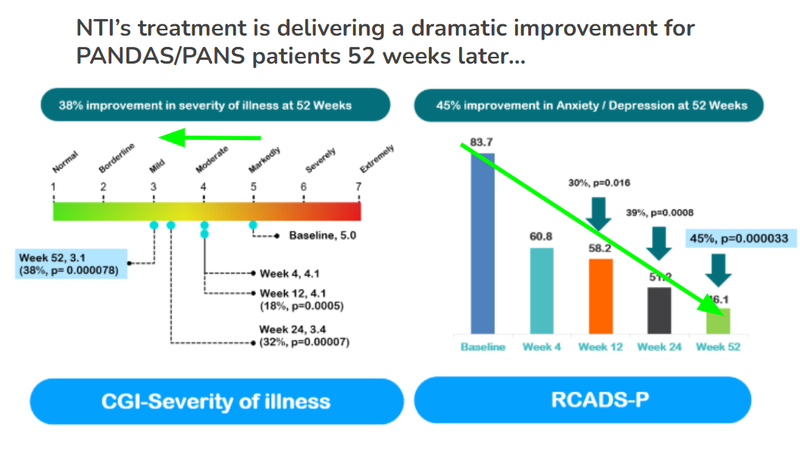
- Continued improvement well after meeting primary endpoint - across two measures of the severity of illness, NTI’s treatment continues to deliver a strong positive impact - on one measure a 38% improvement and on another measure a 45% improvement.
- Caregivers are strongly supportive of the treatment - Today’s announcement included quotes from caregivers of PANDAS/PANS children which provided a glowing endorsement of the impact that NTI’s treatment has had on their children. The caregivers described dramatic life enhancing benefits since their children had been on NTI’s treatment.
- Large potential market opportunity - US$1.2BN. No FDA/EMA approved drug. Rare disorder - potential orphan drug designation could make NTI’s treatments very valuable.
PANDAS (Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections) and PANS (Paediatric Acute-onset Neuropsychiatric Syndrome) results in sudden changes in behaviour and mood.
PANDAS is specifically linked to strep infections, however PANS can be caused by various infections.
- Causes: PANDAS is thought to be triggered by strep infections in children. Whereas, PANS can be set off by various infections, toxins, and other triggers. Both disorders are autoimmune in nature, where the child’s own cells mistakenly attack healthy brain cells.
- Symptoms: Sudden onset of OCD, tics, anxiety, emotional lability and some motor abnormalities. Symptoms may worsen acutely.
- Prevalence: Research suggests that PANDAS impacts 1 in every 200 children, however prevalence of PANS is less clear.
- Treatments: There is no simple treatment for PANDAS/PANS. Treatment usually involves antibiotics, anti-inflammatories and immunotherapy.
It’s a nasty, debilitating condition that impacts both patients and caregivers alike.
Which is why we are so pleased with today’s results and its potential to provide an effective treatment for the condition.
(Strong results like today also enhance NTI’s future case for commercialisation as well)
One caregiver was quoted today as saying: “He is now building a miniature boat - this is something we could never even imagine. Prior to starting the treatment, he wasn’t able to sit on his own for more than 10 mins. We are so grateful.”
We think caregivers are likely very pleased with how NTI’s treatment is tolerated - no adverse events were recorded throughout the 52 week period.
Which is further evidence to us that NTI’s treatment has an exceptionally strong safety profile - something that regulators look upon favourably.
We first covered the very strong results for NTI’s Phase I/II clinical trial in October of last year:

NTI’s clinical trial meets primary endpoints for neurological disease
What’s next for NTI?
There’s plenty to come for the rest of the year:
Meeting outcome - TGA Regulatory Advice
Metabologenomic data from Phase I/II PANDAS/PANS Clinical Trial
Orphan Drug Designation Europe - Rett Syndrome
Orphan Drug Designation Europe - PANDAS/PANS
Orphan Drug Designation USA - Rett Syndrome
Orphan Drug Designation USA - PANDAS/PANS
Completion of Patient Recruitment Phase I/II Cerebral Palsy
Commence Phase I/II Cerebral Palsy Trial
FDA IND/EMA toxicology
Presentation of Phase I/II Rett Syndrome data at international Rett Syndrome conference
For a full description of these upcoming NTI milestones read our latest NTI note:

NTI’s Phase I/II Rett Syndrome clinical trial results improve upon $2.4BN capped Neuren’s results…






