ALA raises $4M and launches $1M Share Purchase Plan
Our cell therapy biotech Investment Arovella Therapeutics Ltd (ASX:ALA) just raised $4M in capital at 4.5c per share.
Off the back of the raise ALA also launched a Share Purchase Plan (SPP) to raise a further $1M from existing shareholders.
The SPP will be done at the same price as the placement (4.5c per share).
The timetable for the offer is as follows:
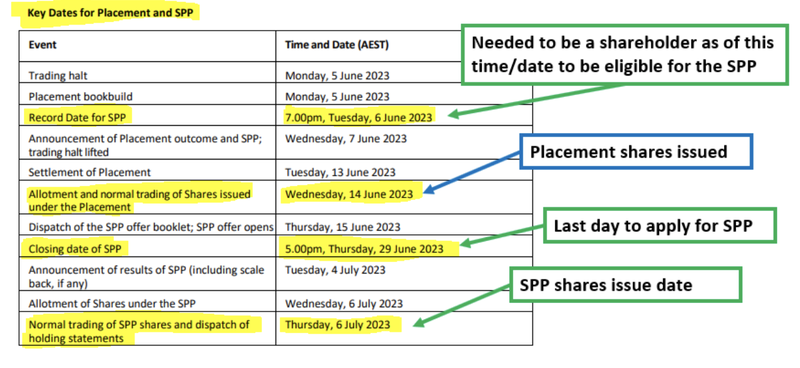
Good time to do a capital raise
ALA started 2023 with a share price of ~2c per share before hitting ~10.5c per share in late April.
ALA is making the most of the re-rate in the company’s share price and raising at an almost 150% premium to its share price from the start of the year.
Sometimes companies watch their share prices go up and then come all the way back down before doing a raise so we like that ALA has decided to make the most of the interest in the company and put away a capital raise.
Especially in the current market where getting funding for biotechs isn't easy.
ALA’s capital raise also comes at a time when the Australian Therapeutic Goods Administration approved the first CAR T Cell cancer therapy for a common cancer.

Although this is a massive breakthrough for the industry, particularly in Australia, the CAR T therapy has not yet been approved by the Medical Services Advisory Committee for taxpayer-funded subsidisation, and costs around $600,000 per patient.
This is where ALA’s comes in as a potential off-the-shelf solution that is more affordable to both the patient and the government subsidising the treatment.
ALA’s Cell Therapy tech is based on iNKT cells (invariant Natural Killer T cells) which are a stronger, more specialised part of the body's immune defences.
There are only a handful of iNKT cell therapy companies globally, one of which is ALA.
Amongst many cell therapy transactions, the only recent iNKT cell therapy transaction (Gilead + Appia Bio) delivered the largest recent deal size - US$875M.
That US$875M transaction was for a research collaboration on a treatment at a pre-clinical stage (the same stage ALA is at now...).
After the cap raise shares are issued ALA will be capped at just $38M.
What’s next for ALA?
The key thing that we are looking out for with ALA is to complete the manufacturing process for its iNKT cell therapy, this is a major milestone on the path to a Phase 1 clinical trial.
In the meantime, ALA has partnered with Imugene to undertake a preclinical study to see if ALA’s treatment in combination with Imogene's product is appropriate for targeting solid tumours.
This proof of concept program aims to:
- Prove the most promising treatment works in a test tube ✅ - ALA and Imugene have already done this - see our Quick Take on that news here: Arovella and Imugene therapy kills cancer in a test tube
- Prove the most promising treatment works in mice 🔄 - The next step after test tube studies, this will see how the treatment works and if it is safe in a living organism (In vitro - mice - studies)
- Take the therapy to a Phase 1 clinical trial 🔲 - After all the pre-clinical data is gathered, ALA and Imugene can choose to progress the therapy into phase 1 clinical trials.






