ALA completes key manufacturing milestone
Our biotech Investment Arovella Therapeutics (ASX: ALA), just took another step closer to clinical studies for cancer cell therapy treatments.
Today, ALA confirmed that a “Good Manufacturing Practice” (GMP) grade lentiviral vector has been manufactured and passed through quality screening for its ALA-101 treatment.
ALA-101 is the company's treatment for lymphomas and leukemias.
Today’s news is especially important because the lentiviral vector is one of the most important manufacturing steps for ALA’s treatment - the lentiviral vector is an input programmed to target and eliminate cancer cells.
After ALA completes the clinical manufacturing process, it will be focused on getting its iNKT cell therapy into Phase 1 clinical trials.
ALA has previously confirmed in its latest video, that it expects Phase 1 human trials to occur in the second half of 2024.
🔲 Phase 1 clinical trial for ALA-101
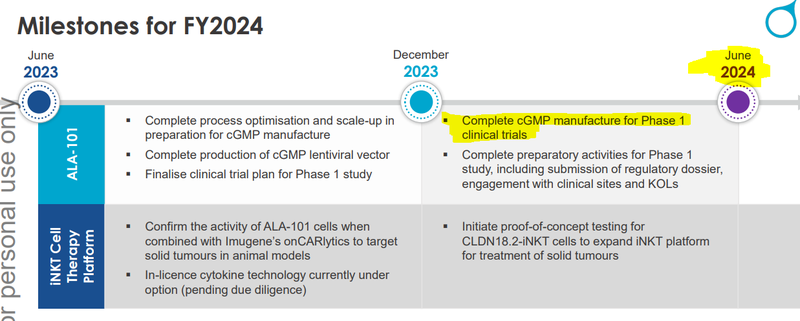
We set a series of milestones we wanted to see ALA tick off in the lead up to clinical trials.
Today’s news came from the first set of those milestones (manufacturing progress).
These are the sub-milestones we are looking for from ALA in the lead up to what we hope is getting its treatment into the clinic in the second half of this year:
- 🔄 Further manufacturing milestones (optimisation, scale up and manufacture of its lentiviral vector) - ALA needs to improve the process to make enough of the therapy to enter a trial, and the lentiviral vector can be thought of as the delivery system for the therapy internal to the body.
- 🔲 Finalise clinical trial plan for Phase 1 study
- 🔲 Take the therapy to a Phase 1 clinical trial - After all the pre-clinical data is gathered, ALA can choose to progress the therapy into Phase 1 clinical trials.
What’s next for ALA?
In our last ALA note, we touched on all of the key bits of newsflow we want to see from the company this year.
Check out our note here - ALA is our best performing stock in 2023 - here’s why, and what to expect in 2024






