EMD up 37% on no news yesterday… MDMA Therapy for First Responders
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 5,866,667 EMD shares and 2,933,334 EMD options at the time of publishing this article. The Company has been engaged by EMD to share our commentary on the progress of our Investment in EMD over time.
Yesterday, our biotech investment Emyria (ASX:EMD) was up 37% on no news.
EMD is delivering and developing new treatments to improve mental health.
EMD is focussed on psychedelic assisted therapies, in particular MDMA-assisted therapy for PTSD.
EMD is a first mover in the space globally, taking advantage of Australia being the first country in the world to legalise the use of psychedelics for treatment of mental health disorders.
EMD has a unique status as one of the few organisations in Australia approved by the Therapeutic Goods Administration (TGA) to use MDMA for therapeutic purposes.
And it is already treating patients suffering from PTSD in clinics in WA, generating clinical billings of over ~ $1M a quarter.
So why did the EMD share price start running yesterday?
Here is what we know so far:
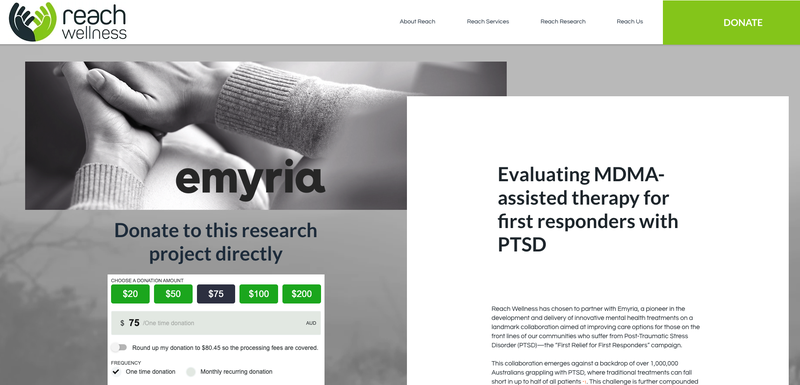
- Early yesterday morning, Mental health organisation “Reach Wellness” published a page on its website detailing that it had chosen to partner with EMD to support 50 first responders suffering with PTSD to use EMD’s treatment.
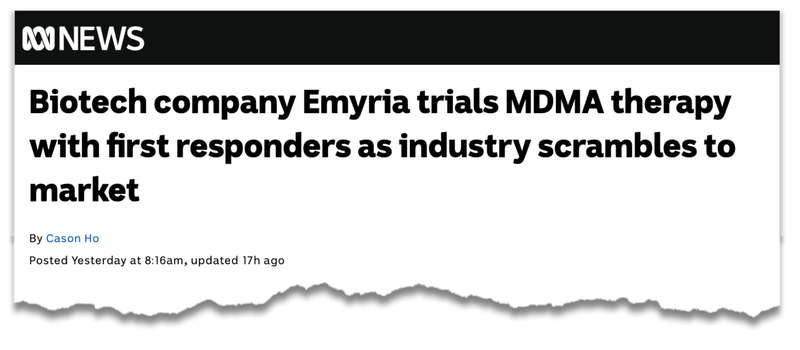
- At 8:16am yesterday, ABC News published a feature article on its website detailing the partnership between EMD and Reach Wellness, titled: Biotech company Emyria trials MDMA therapy with first responders as industry scrambles to market
- Shortly after, the ABC published a 2 minute 49 second video report about EMD on its Youtube channel, with ABC confirming that the goal of the partnership was for Reach Wellness to fund 50 EMD treatments at $30,000 per treatment, which equals $1.5M to EMD.
- At 7:00pm AEDT last night the same ABC video segment that was published to Youtube was shown on national TV.
Aside from the ABC segment being shown on broadcast TV last night, the rest of the above occurred during market open hours yesterday.
A key catalyst is for EMD to prove that their treatment can be externally funded for its patients, either by insurance companies or the government (“payers”).
This partnership with Reach Wellness is a small but early proof point on the “payer” model we want to see EMD prove and scale.
Just now, EMD responded to a price and volume query from the ASX, confirming the reason for the share price run was the attention driven by the ABC news story (ASX announcement).
Many small cap biotech stocks have been on a great run the last few months - in our Portfolio DXB, NTI and ALA have been standout performers.
Until yesterday EMD, from a share price perspective, didn't seem to get the memo yet that 2024 is a strong year for small cap biotechs.
But it looks like the mainstream media coverage yesterday gave it a kick start that we hope will be a continued and sustained period of share price growth for the company.
In parallel with the ABC coverage, Reach Wellness published a “Research Donation” page on its website detailing that it had chosen to partner with Emyria to support first responders suffering with PTSD, inviting donors to help fund the $1.5M project.
(Source)
We decided to help get the ball rolling with a donation.
We think it is a great initiative for all the stakeholders involved and especially for the first responders, who do a great job in tough conditions, and get exposed to some very hard and traumatising situations on a daily basis (think paramedics, police and firefighters).
Anyone can donate here.
Here is the ABC feature on its website on EMD:
(Source)
In the ABC feature article was a video that highlighted (at 1:23 seconds) that “Reach Wellness had pledged to fund this clinical trial up to the first 50 patients”.

EMD is currently running a clinical trial for MDMA-assisted therapy to show that MDMA-assisted therapy is better than the current standard of care when it comes to treating people with PTSD.
This clinical trial has begun and EMD will treat up to 72 patients.
The cost of each treatment to the patient is A$30,000.
If 50 patients are funded by Reach Wellness, it could be up to $1.5M for EMD through this charity drive.
As we noted above, EMD published a response to the price query this morning highlighting that a collaboration exists between Reach Wellness and EMD.
BUT, it detailed that the material terms are yet agreed, and that the nature of the collaboration is not yet finalised.
So who is Reach Wellness and what is this charity drive about?
Reach Wellness is a not-for-profit organisation focused on delivering solutions for the treatment and support for mental and physical health for first responders.
Think emergency services, paramedics, fire brigade etc...
These are all people, who because of their vocation, are susceptible to stressful situations and have treatment resistant PTSD.
There is a growing need for mental health services in Australia.
Up until recently, Reach Wellness' members had been utilising the Bethesda Clinic, offering important mental health services with a specialised wing for first responders.
However, unfortunately last month the Bethesda Clinic in Perth shut down due to “lack of psychiatrists willing to admit in-patients under their care and insufficient private health rebates to cover their service.”

(Source)
With the clinic shut down, Reach Wellness needed to find an answer to support mental health services for its members - in particular those suffering with PTSD...
Hence the collaboration with EMD.
As we noted above, Reach Wellness has pledged to fund up to 50 patients in the EMD trial.
This could be the first “payer agreement” that EMD has secured for its trial.
At $30,000 patients, this agreement could be worth up to $1.5M.
Again, you can see the donation page for Reach Wellness is here:

One of the Key Objectives we wanted to see from EMD was to secure a “Payer Agreement” with a large insurance company or government, to cover the cost of care for patients.
We think that this collaboration with Reach Wellness is the very first step towards this objective.
It is the first proof point that a third party group is willing to pay for EMD’s treatment on behalf of their members.
With Reach Wellness stepping in, we may see other institutions like governments, insurance companies or other large member organisations come to the table.
This brings us to our Big Bet for EMD:
Our EMD Big Bet
EMD re-rates to a +$300M market cap by making a breakthrough with one or more of its clinical trial programs (MDMA, psilocybin, ketamine, CBD) while rapidly growing the footprint of its mental health treatment clinics.
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our EMD Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
What does EMD do?
EMD is an “integrated clinical drug development and care delivery” company.
EMD’s primary area of focus is on psychedelics such MDMA, psilocybin and, more recently, ketamine.
The company is looking to use these drugs to support treatments for mental health problems- first targeting PTSD, and then expanding into other areas.
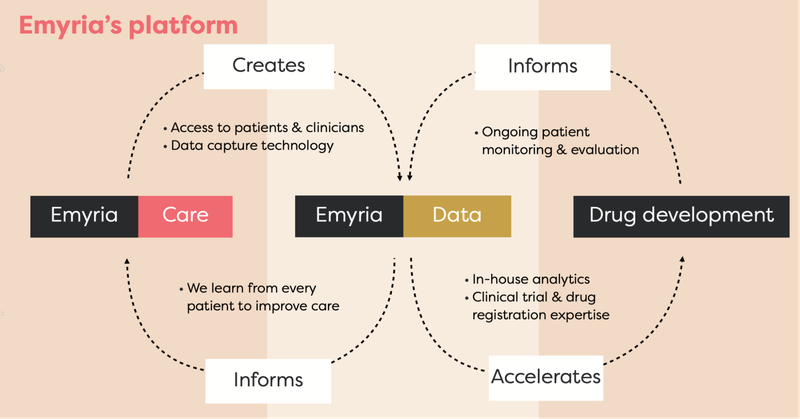
EMD has an integrated business strategy because it has:
- Treatment centres: Places where treatments can be administered with wrap-around care.
- Treatments: EMD can legally source the drugs, which include psilocybin, ketamine, MDMA and an ongoing drug development program into analogues, aiming to make the treatment work better for a range of conditions.
- Data: Through its clinics and approved clinical trials, EMD collects and owns valuable patient data to help improve its drug treatments and therapy programs.
- Clinical drug development: EMD is developing its own MDMA analogues to harness MDMA drug treatments.
What is EMD’s business strategy?
As we noted above, EMD is an integrated clinical drug development and care delivery company.
This basically means that EMD:
- Owns physical clinics,
- Provides care for patients, and;
- Has the infrastructure to develop the means of delivering assisted psychotherapy treatments.
Late last year, we visited the Pax Centre in Perth and met Emyria’s lead psychiatrist Dr John Laughrane. It was great to chat to him to understand the potential of the treatments he is offering, and how the process works.

The drugs EMD is focused on are MDMA, psilocybin and ketamine.
EMD’s first PTSD trial is currently ongoing with the first patient commencing their trial last year.
The company’s commercial strategy sits around two key areas:
- Scale up the ability to deliver care
- Secure a payer for treatment
These are the two key goals that EMD is working towards and if both are achieved, we expect the company to be a significant player in this emerging industry.
There are very few ASX-listed companies leveraged to the psychedelic space, and we think that EMD is primed to take advantage.
So on that note, let’s take a look at how EMD will get there...
Part A: Scale up the ability to deliver care
First EMD will be looking to scale up its ability to deliver care.
Right now, EMD can deliver commercial MDMA-assisted therapy for PTSD, but it can only do it on a small scale.
It has enough MDMA to treat 72 patients (in its clinical trial), and has only one physician authorised to prescribe MDMA assisted therapy outside of a clinical trial.
Since we first Invested in EMD the company has ticked off a few key milestones to achieve “scale up” of its assisted therapy offering.
Firstly, EMD hired Greg Hutchinson as Chairman in November last year to help scale up its business.
Greg is the CEO of Sonic HealthPlus, who has become the largest provider of occupational and community medical services in Australia.
And Greg is a gun at scaling up businesses.
Parent company Sonic Healthcare is now a $13.4BN company:
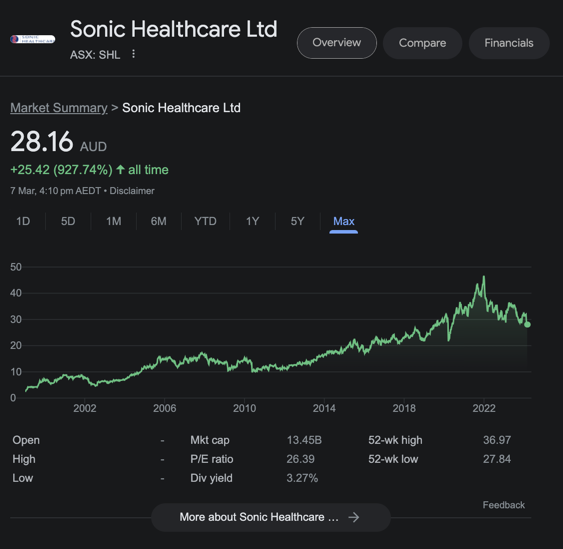
To read more about the hire: EMD first mover in psychedelic care market
Secondly, EMD achieved “Authorised Prescriber Status” for one of its psychiatrists last month.
This allows EMD, through its psychiatrist, to provide MDMA assisted-therapy OUTSIDE of a clinical setting.
There are very few people in Australia who have this status, you can read more about the news here: APPROVED: EMD Receives Authorisation to Prescribe MDMA for Therapy.
But in order for EMD to continue to scale there are a number of milestones it will need to achieve:
- More staff / contractors with approved AP Status
Getting to Authorised Prescriber Status is no easy task...
It requires multiple submissions to the TGA, ethics approval, a tried and tested protocol for therapy delivery and strict guidelines on how the treatment can be administered.
Because EMD has done this before, we expect the company will be able to do it more efficiently with other physicians on its books and scaling up its MDMA-assisted therapy offering.
Also, any clinicians that want to work in the space or want to get themselves listed as “Authorised Prescribers”, may be able to come to EMD to licence its blueprint/protocol for MDMA assisted therapy.
- More MDMA Product
MDMA is a heavily controlled substance.
In order for EMD to run assisted therapy sessions at scale it will need more of the drug for the treatment.
Securing MDMA supply will be crucial for EMD’s scale-up efforts.
- Treatment approval for more indications
EMD currently has approval just for MDMA-assisted therapy for PTSD.
In order to help more people with different mental health issues (like depression, anxiety, etc...) EMD will need to secure specific approvals for each type of treatment.
Also, if EMD applies different drugs, like psilocybin or ketamine, it will need approval for those as well.
- More clinics
The long term vision for EMD is to be like a franchise for mental health services. It will need more clinics with more access to patients to scale up its services.
We think this is a big part of EMD bringing Greg Hutchinson from Sonic Healthcare Plus on board - this is exactly what he did there.
These are some of the key milestones that we will be looking out for to see EMD “scale up” its business.
But we think that the company is on the right track in order to execute on this plan.
Part B: Secure Payer for Treatment
If EMD can scale its services, that will be a massive achievement.
However, the cost of treatment can be expensive for people out-of-pocket.
Therefore, EMD will likely be looking to secure a Payer Agreement, whereby an insurance company or government body covers the cost of the treatment.
Yesterday’s breaking news by the ABC that Reach Wellness has pledged to fund up to 50 patients is a strong indication of the potential for EMD to secure payers for its treatment.
What organisations like insurance companies and governments want to see is a cost benefit analysis on funding the treatment versus the current standard of care.
Based on the most recent data published by Lykos Therapeutics, MDMA assisted therapy is 50% of the cost to treat PTSD as well as more effective in the early stages:
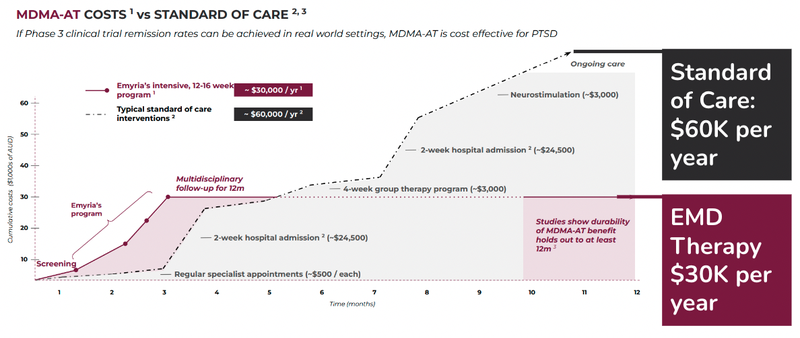
Just last month Lykos Therapeutics, locked away $100M in funding to support its late-stage trials for PTSD.
Payers will likely want to see lots of clinical data that show effectiveness, which is why EMD is conducting its clinical trial for MDMA-assisted therapy on PTSD.
This trial is for EMD to develop its own proprietary set of data to prove that its treatment protocol is safe, effective and more efficient than the current standard of care.
Last year, Lykos Therapeutics published results of an MDMA-assisted therapy Phase 3 trial:
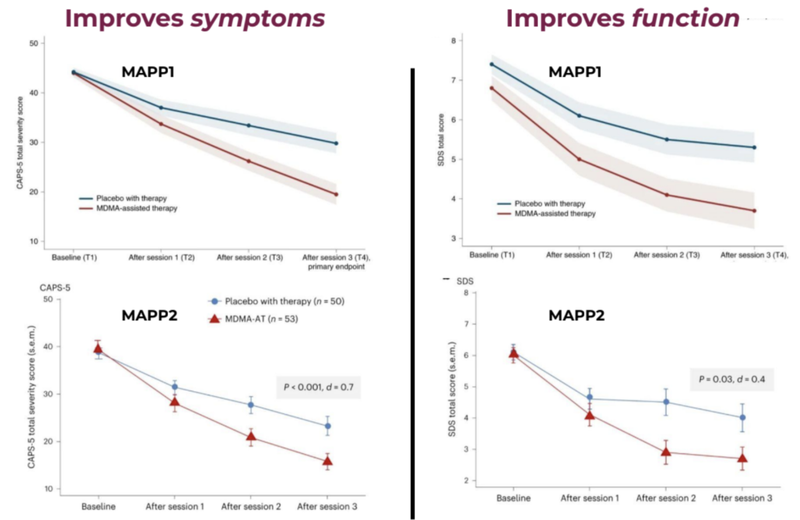
The blue line shows the placebo group and the red line shows the Assisted Therapy group with improvements in function and symptoms.
A key stat from these trials is that 71% of MDMA assisted therapy participants no longer met the criteria for PTSD compared to just 47% from the placebo group.
There is a real demand for funding for mental health services - and we think that EMD could tap into this funding.
What does success look like for EMD?
If EMD is able to do both (A) Scale up its business and (B) Secure Payer Agreements, we think this will create a significant moat around the business.
An economic moat is a competitive advantage that a company possesses which is very difficult to copy or replicate.
For EMD, if it is able to achieve its goals it will have the facilities, industry contacts, knowhow and licence to capture a big share of this emerging market.
Without other players able to easily enter the space...
Currently EMD is targeting PTSD, but it could target other mental health issues like anxiety, depression etc...
The moat that EMD possesses is its access to clinics and its already approved protocol for Authorised Prescriber status in Australia.
EMD will have a first mover advantage, and if it is able to scale up and secure payers for its treatment it will prove to be a strong success.
What is next for EMD?
Finalise Funding Deal with Reach Wellness 🔄
Although the ABC stated that Reach Wellness had a collaboration with EMD to fund up to 50 patients, EMD has said that it is awaiting a binding agreement.
MDMA-AT Phase 2b patient recruitment updates 🔄
We want to see EMD recruit all 72 patients into the trial so that we will have a timeframe for results.
These results will show how effective EMD’s protocol is at treating PTSD and will support commercial negotiations with payers to cover the cost of MDMA-Assisted Therapies for its members.
Approval for ketamine assisted therapy protocol 🔄
EMD has a pending application for a ketamine assisted therapy trial.
Secure key approvals for psilocybin use 🔄
EMD has a pending application for psilocybin use in an assisted therapy trial.
Secure a major payer partnership (we see this as a major catalyst) 🔲
If EMD is able to secure a payer for its treatments, we think this would be a major catalyst for the company.
Key Risks for EMD
Clinical Trial Risk
There is no guarantee that EMD will be able to show that its Assisted Therapy programs will be more successful than a placebo in a clinical setting.
Regulatory Risk
EMD is working in a heavily regulated space.
The company will have strict guardrails on what it can and can’t do thus limiting the speed at which it can move and potential innovation.
Funding Risk
Small caps often need to raise cash to fund their growth.
Whilst EMD is generating revenue now, it is still making a loss - i.e. it spends more cash than it brings in.
If EMD is unable to develop a self-sustaining business model with positive operating cash flow, this could require EMD to raise capital in the future, likely at a discount to market prices to secure funds.
Our EMD Investment Memo
In our EMD Investment Memo, you can find:
- EMD’s macro thematic
- Why we Invested in EMD
- Our EMD “Big Bet” - what we think the upside Investment case for EMD is
- The key objectives we want to see EMD achieve
- The key risks to our Investment thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

