FDA knocks back approval for MDMA-assisted therapy
On Friday the US FDA knocked back approval for Lykos Therapeutics MDMA-assisted therapy to treat PTSD.

This application would have marked the first FDA approved therapy for MDMA and is a setback to the industry.
The FDA didn’t reject the trial, but instead requested the company undertake another phase 3 trial.
Despite promising results, it looks like the FDA is taking a conservative approach to approvals which is understandable given the elicit nature of the drugs as well as the potential ‘floodgates’ that could open up.
This decision does however open up an opportunity for our psychedelic therapy company Emeryia (ASX:EMD) who is developing its own MDMA-assisted therapy and conducting a clinical trial right now in Australia.
In an email to shareholders EMD addressed the FDA filings, here are our key takeaways:
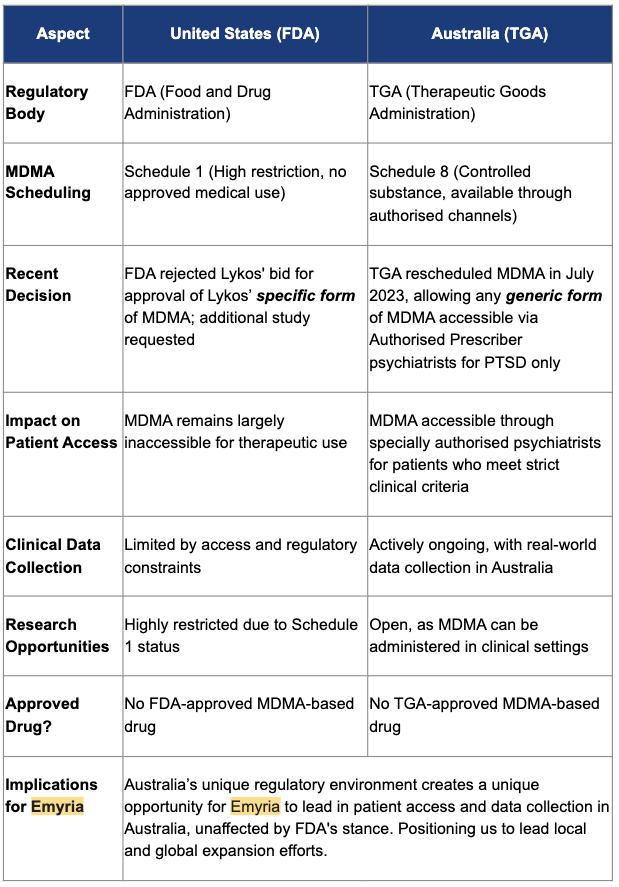
- The FDA decision will inevitably delay MDMA-assisted therapy in the US
- The US is not Australia, and Australia has a much friendlier regulatory environment for MDMA-assisted therapies
- This decision provides EMD will an opportunity to break ground and set a benchmark in the industry within a jurisdiction with an easier pathway towards commercialisation
- The decision doesn’t affect any payer (commercialisation) discussions for EMD for use of its therapy within Australia.
- EMD has the chance to “set the benchmark” for this kind of therapy, positing it as one of the most advanced opportunities for investors looking to be leveraged to the thematic.
We think that this was very good response from EMD and addressed some of the concerns and opportunities from this decision.
In the update EMD also stated that it has recruited 12 patients (of 72 planned) into its own MDMA-assisted therapy program.
Last week one of our analysts visited the EMPAX Centre where all of the MDMA-assisted therapy is done.
Here is the excerpt from last weeks “weekender” note.
Finally, we made a trip to the Empax centre which is the clinic that our psychedelic assisted therapy Investment Emyria (ASX:EMD) has recently acquired.
It’s a state of the art facility and EMD Managing Director Michael Winlo was kind enough to give us a tour.
Of particular note to us, was the demonstration of EMD’s AI generated music which provides patients with the right music at the right time to elicit the right assisted therapy experience.
EMD is treating patients with serious PTSD conditions, and PTSD is a condition which is characterised by a close association with extremely painful memories, as well as causing short and long term memory loss as well (Source).
There are over 1 million people with PTSD in Australia (Source).
And given recent research that music and memory are very closely linked (and can help with Alzheimer’s - Source), we think EMD’s AI generated music could have a profound beneficial effect on what we hope is a growing number of patients.
EMD has also been in the news recently, with the AFR publishing a deep dive on the company: Psychedelics as a serious investment? These billionaires think so.
We recommend giving it a read…

How does the FDA news affect our EMD investment memo?
Ultimately, this news affects two key risks forecast for EMD.
“Regulatory risk” has increased while “competition risk” has decreased.
This decision by the FDA affects the regulatory environment in the US but not in Australia.
EMD does have plans to grow into the US at some point in the future, but this decision may put those on pause for now.
Regulatory risk.
The regulatory changes that allow EMD to operate in the field of psychedelic therapies are new and could be reversed. A regulator could step in and intervene either across the industry or specifically in relation to EMD. A change of government could also bring about a regulatory reversal.
Source: What could go wrong? EMD Investment Memo 4th September 2023.
Lykos Therapeutics was the industry leader in MDMA-assisted therapy, with the most advanced program.
This decision opens up the opportunity for EMD to move into pole position as a global leader in the psychedelic therapy space.
Investors that are looking to advance MDMA and psychedelic assisted therapy products may turn to EMD as an alternative (or in addition to) Lykos Therapeutics, given that EMD is based in Australia which has a friendlier regulatory environment.
Competition Risk
EMD will need to move quickly to establish its presence in the market. If progress is slow, another care provider or alternative treatments could emerge hurting EMD’s prospects.
Source: What could go wrong? EMD Investment Memo 4th September 2023.
EMD provided a detailed summary of the difference between the US and the Australian regulatory environment.
In particular the “Research Opportunities” in Australia are open, which means that they are not as highly restricted as they are in the US.
We think that this gives EMD a distinct advantage here in advancing its product.