EMD partners with University of WA on novel Serotonin-Releasing Agent
Yesterday EMD announced that it has secured a ~$500,000 grant from the WA government to advance pre-clinical (animal) studies on two key MDMA analogue products.
These products help the brain regulate serotonin, one of the most important brain functions.
EMD’s product addresses two of the key challenges with MDMA for assisted therapy including:
1. MDMA takes a long time for the body to process
MDMA has a long half-life, which is a measure of the length of time that it takes for a chemical to be broken down.
A longer half-life means that the effects of the drug will continue over an extended period of time.
For a therapy session, this is not ideal as clinicians will need to be working with the patient for as long as the chemical is still active.
Reducing the half-life reduces the therapy time and the cost of clinicians.
2. MDMA is euphoric, so it is open for abuse
One of the challenges with MDMA-assisted therapy is the abuse potential due to the euphoric effects of the drug.
If EMD is able to create an analogue that reduces the euphoric effect while maintaining the therapeutic effects it will address one of the main concerns over the drug.
EMD has secured $500K from the WA Government to advance these analogues with results from pre-clinical trials due in early January.
This directly impacts our second objective for EMD:
Objective #2: Develop novel MDMA treatments
We expect EMD to continue its clinical trial work and drug development, which includes preclinical tests on MDMA analogues (animal studies) for faster acting MDMA and MDMA “without the high” for Parkinson’s disease.
Milestones
🔄 Pre-clinical study results for fast acting MDMA analogue 🔲 ➡ 🔄
🔲 Pre-clinical study results for Parkinson’s MDMA analogue
🔲 Identify lead drug candidates for Phase 1 trial
Source: Objectives: EMD Investment Memo 4th September 2023.
The treatment is also said to help with neurological conditions such as Parkinson’s disease. Which could be a lucrative opportunity if it is proven to work.
EMD says that the results of the pre-clinical studies are due early next year.
Canadian psychedelics company up 1,446% in one day
Two weeks ago Canadian listed psychedelics company Bright Mind Biosciences increased 1,446% in one day… on no news.
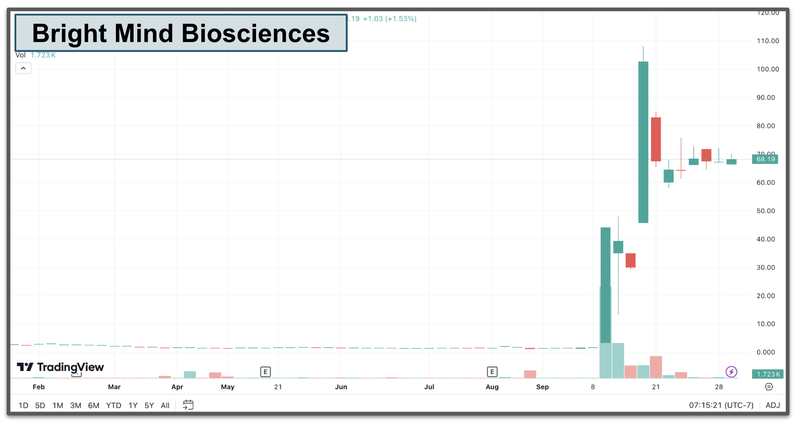
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
Bright Mind Biosciences is developing a product that selectively activates or blocks serotonin receptors in the brain.
Serotonin is the most prominent neurotransmitter in the brain and the molecule modulates many functions.
Bright Mind identified that if it could modulate the release of serotonin it could have a therapeutic effect (specifically in treating epilepsy) through the use of various psychedelic drugs like ketamine, MDMA and psilocybin.
The reason for the interest in the company was that the day before its competitor Longboard was acquired for $2.6Bn by Lundbeck.
(Source)
Longboard is also developing a treatment that selectively targets serotonin receptors in the brain.
The company was well advanced (at Phase III) for its treatment of rare epilepsy conditions.
However, these two companies, Longboard that was acquired for $2.6Bn and Bright Mind that went up 1,446% in one day, indicates the interest in drugs that moderate serotonin.
This is the exact type of drug that EMD is looking to develop with the University of WA.
We see this as a “free hit” for EMD given that the pre-clinical studies will be funded by the WA Government.
It’s important to note that compared to both Longboard and Bright Mind, EMD is at a very early stage when it comes to drug development.
However, if EMD is able to advance its MDMA analogues that specifically it could potentially alleviate some of the challenges of the treatment, OR potentially open up the possibility of new drug development avenues.
That said, we still see this part of EMD’s business as a “side bet”.
The main game of scaling up its offering of MDMA-assisted therapy and partnering with a Payer to secure funding for the treatment.
EMD expects results from its pre-clinical trial to be complete at sometime in early 2025.






