$15M grant for psychedelics studies as calls to combat mental health intensify
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Australia’s federal government will grant $15M to clinical studies that explore the use of psychedelic drugs like magic mushrooms and ecstasy, to treat Australians with debilitating mental illnesses.
This is a crucial breakthrough in the fight against mental illness, with almost half of Australia’s adults set to face mental health challenges during their lives.
According to Black Dog Institute, “1 in 5 of us will experience symptoms of mental illness in any given year. In Australia that’s around 5 million people. And roughly 60% of these people won’t seek help.
Suicide was the main cause of death for Australians aged 15 to 49 years in 2019, an issue that in many cases could have been prevented had people felt comfortable in seeking help or alternative therapies.
Mental health issues were further exacerbated during COVID-19 lockdowns, with isolation causing anxiousness, stress and worry and worsening existing mental health conditions.
A survey conducted by Sapien Labs found showed that 57% of respondents experienced some COVID-19-related adversity or trauma. The survey was conducted in eight English-speaking countries and included 49,000 adults.
"The data suggest that there will be long-term fallout from the pandemic on the mental health front," Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
With worsening mental health conditions, the government grant comes as welcome news.
Thus far, there have been few pharmaceutical breakthroughs to combat the problem.
However that looks set to change.
As The Australian recently reported, “the federal government has seized on strong evidence emerging internationally on the potential treatment of mental illnesses that are resistant to first-line treatments with substances such as ketamine, psilocybin (magic mushrooms) and MDMA, commonly known as ecstasy.
Evidence points to some treatment success using psychedelics in conjunction with psychological or psychiatric care.
In fact, the US Food and Drug Administration has granted “breakthrough therapy” status for the use of magic mushrooms to treat mental health disorders.
Australia’s Health Minister Greg Hunt wants to ensure his country is at the forefront of innovation in this space.
“The early results of trials in Australia and internationally are extremely encouraging, but more research is desperately needed before these approaches can be used by psychiatrists outside of controlled clinical trials,” Mr Hunt said.
“It is vital that we continue to support the search for new and better treatments for mental illness.
“This grant opportunity will boost local research into potentially lifesaving therapies and offers hope all those suffering from mental illness, including our veterans and emergency service personnel dealing with the devastating effects of PTSD.”
Click on the following Channel 9 report to learn more:

Research into the effects of psychedelics treatment for mental health illnesses has the backing of former Liberal Party director and trade minister Andrew Robb.
Robb has been suffering from diurnal depression for over 50 years.
This type of depression "means you go to sleep taking on the world, and you wake up deeply depressed".
"It's very hard to accept it. In fact, I didn't accept it for myself for decades that I had a depressive condition. I kept putting it down to all sorts of other things," Mr Robb told 7.30.
"It's very difficult to explain the nature of depression. It defies logic. In fact, what it does do though, is it sucks the joy of life out of the individual. You see everything in a negative fashion.
"It was hard on my family too, because I was a non-event for the first few hours a day. In fact, my wife used to say that to people. We don't talk about the state of our marriage before nine o'clock."
Mr Robb is now on the board of Mind Medicine Australia, which advocates for psychedelic therapy, currently available in Canada, the US, Switzerland and Israel.
"What we've seen is over 150 clinical trials take place. In the last 20 years, we've seen over 3,000 people, patients, involved in these trials, and the results are spectacular," Mr Robb said.
"They're getting up to 60 to 80 per cent remission. This is not relief, this is remission.
"Now, those sort of results are not possible [with current medications] ... At best, 30 per cent of people in Australia will respond to the antidepressants. So you get 70 per cent who are not responding. And we need to have alternatives."
Next month TGA will decide whether to allow psilocybin and MDMA to be downgraded from schedule 9 (prohibited substance) to schedule 8 (controlled drug).
It should be noted that in a meeting of the TGA earlier this year, this decision was rejected.
Psychedelics on the ASX
As Finfeed readers would know, Creso Pharma (ASX: CPH) became the first ASX listed company to acquire 100% of a psychedics company
CPH will acquire Canada based Halucenex Life Sciences Inc., an established psychedelics company developing treatments for a range of mental illnesses.
Halucenex is targeting two specific verticals:
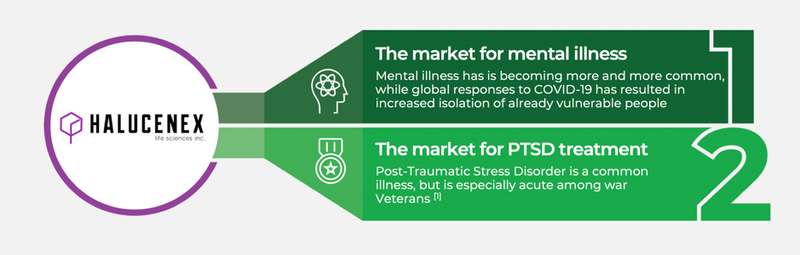
It also has access to one of the largest supplies of synthetic psilocybin in Canada.
The company’s focus on PTSD is critical, as up to 12 per cent of Australians experience PTSD during their lifetime.
Currently, Halucenex has a strong affiliation with Canadian Veterans that should provide a fast track to early revenues.
As we reported yesterday, Halucenex has appointed True North Clinical Research as the principal investigator for its proposed Phase II Clinical Trial to test the efficacy of psilocybin for the treatment of resistant Post Traumatic Stress Disorder (PTSD) in veterans and first responders.
These trials could have global ramifications.
Game changing acquisition with far reaching consequences
In the following Q&A session, CPH explains why it is moving forward the Halucenex acquisition.
Q. What is Halucenex?
A. Halucenex is an established life sciences development company focused on researching, developing and licencing novel psychedelic molecules for the global pharmaceutical and nutraceutical markets. Halucenex is currently progressing clinical trials to research the efficacy of psilocybin used in psychedelic-assisted psychotherapy (PAP) to treat and alleviate Treatment Resistant Depression and other mental illnesses.
Q. What is Psychedelic-Assisted Psychotherapy (PAP)?
A. Psychedelic-assisted psychotherapy refers to therapeutic practices that involve the ingestion of a psychedelic drug.
Q. What is psilocybin?
A. Psilocybin is the active chemical found in magic mushrooms, which has been shown to have considerable benefits for mental health patients. It is very scarce and commands high prices.
Q. How does Halucenex guarantee supply of psilocybin?
A. The growing demand for medical-grade psilocybin to conduct research and clinical trials and the lack of steady, consistent supply chains has led to a supply shortage globally. In anticipation of further shortages in product availability, Halucenex has signed a supply agreement with one of Canada’s pharmaceutical grade psychedelics manufacturers, Psygen Industries Inc., securing supply of 11.6g (11,600mg) of synthetic psilocybin for immediate use in upcoming clinical trials.
This agreement makes Halucenex one of only approximately 11 companies to secure supply from Psygen, and provides the company with one of the largest supplies of synthetic psilocybin in Canada and significantly de-risks future endeavours.
Synthetic psilocybin is preferred over naturally sourced psilocybin in a clinical setting, as naturally sourced psilocybin is known to have greater inconsistency of potency and therefore dosage. Health Canada therefore approves clinical trials only if companies have access to synthetic psilocybin to ensure consistency of dosage and purity in formulations.
Q. Where is Halucenex based?
A. Halucenex is based in Canada. It operates at 6,000 sq. ft. medical treatment facility in Nova Scotia, located next to the Hants Emergency Hospital, with a Controlled Substances laboratory, and 18 treatment rooms dedicated to providing psychedelic-assisted psychotherapy.
Q. What is Halucenex’s core business and strategy?
A. Halucenex is rapidly becoming a fully vertically integrated provider of psilocybin and psychedelic-assisted psychotherapy treatments through a three-tiered strategy.
Halucenex will initially focus on the development of a proprietary synthetic psilocybin formulation to de-risk supply and increase yields for future micro and macro dosing formulations, as well as open the manufacturing vertical.
It will also conduct research into interactions between synthetic psilocybin and natural psychedelic psilocybin mushrooms. This will include cultivation and extraction of multiple psychedelic derivatives with testing to develop intellectual property.
Halucenex is also aiming to become a clinical drug provider of psychedelic-assisted psychotherapy. It has a defined clinical trial schedule and binding supply agreements, which will accelerate a path to commercialisation.
Q. What is the market potential for PAP as an alternative treatment route?
A. The emerging global market for psychedelic medicines is estimated to be worth over US$100Bn. As well as the market generally, Halucenex is targeting the high growth verticals of mental illness and Post Traumatic Stress Disorder (PTSD).
The COVID-19 pandemic has accelerated the urgent need for effective mental health treatments, with lockdowns leading to a significant increase in mental health diagnosis. Currently, there are an estimated 264 million people affected by depression globally[i] and is expected that one in four people will be affected by mental or neurological disorders at some point in their lives.
Increases in traumatic events globally have contributed to the rise of PTSD and it is estimated that the market for therapeutics to treat the condition will grow to over C10.5Bn by 2025.
Recent studies have shown that psychedelic molecules can be used in PAP to treat mental health illnesses such as:
- Depression
- Anxiety
- Substance abuse disorder
- Obsessive Compulsive Disorder (OCD)
Q. What are some of the problems with current treatments for mental health and PTSD?
A. The available treatments for mental health have limited effectiveness and many side effects. Presently, many offerings use a combination of skilled therapists, support groups and medication. There is limited, timely access to these resources in a matter coinciding with patient treatment needs.
Regularly, patients can only access one of these therapies, which lead to a less than satisfactory outcome. This applies for patients with drug-resistant conditions and highlights the pathway for PAP as a new alternative treatment.
Q. What is the current regulatory landscape for psychedelic-assisted psychotherapy?
A. The regulatory landscape for PAP is rapidly shifting and becoming more favourable for Halucenex.
In North America, there have been considerable regulatory developments, which signal a growing acceptance of PAP. These include:
- A designation from the US Food and Drug Administration (FDA) in 2019 and 2020 for 2 companies for psilocybin-assisted psychotherapy as a ‘breakthrough therapy’ for Major Depression and Treatment Resistant Depression
- The Canadian Health Minister granting a growing number of people with terminal cancer access to psilocybin mushrooms through a one-time exemption to Canada’s Controlled Drugs and Substances Act
- The US FDA granting expanded access to MDMA for PSTD patient treatment trials
- Washington DC decriminalising the possession and cultivation of psychedelic plants and fungi
- Oregon, USA legalising psilocybin for the mental health treatment of adults in supervised settings
In Canada, most psychedelics are categorised as Schedule 3 controlled substances. This includes ayahuasca, psilocybin, and LSD. MDMA and ketamine are listed under Schedule 1. The Canadian authority, Health Canada, grants exemptions to companies looking to use psychedelics for a medical or scientific purpose in the form of a Controlled Drugs and Substances License.
Other countries where using psychedelics is legal for either recreational or medicinal purposes include:
- Brazil
- Jamaica
- The Netherlands
Halucencex is confident that there will be a continued shift in the regulatory environment, which will underpin growth for the company.
Q. Why did Creso decide to acquire Halucenex?
A. The acquisition provides Creso Pharma with direct access to the emerging psychedelics medicines market. Creso Pharma believes that the acquisition will allow it to bypass significant barriers to entry into the market, positioning it with an early-mover advantage to bring products to market. The acquisition also marks the beginning of the evolution of the Company from cannabis cultivator, extractor and medicines provider, to integrated natural medicines provider to improve people’s mental health and wellbeing.
Q. What are the expected synergies from the combined operations?
A. The combined group of Creso Pharma and Halucenex provides a number of synergies and growth opportunities.
Creso Pharma expects to fast track Halucenex’s growth through its established global distribution network and partners in the pharmaceuticals space and providing access to the Company’s cutting-edge cannabis cultivation facility in Canada, which can be readily adapted to the cultivation of magic mushrooms. Halucenex will also be able to leverage off the expertise of Creso Pharma’s leading management team, which has significant experience in clinical trials, pharmaceutical product development, and go-to-market know-how.
Together, both parties will be positioned at the forefront of innovation in the psychedelic medicines sector.
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

