ILA appoints new non-executive chairman
Our biotech Investment Island Pharmaceuticals (ASX: ILA) just appointed Jason Caroll as its new non-executive chairman.
ILA is a drug repurposing biotech company targeting infectious diseases.
ILA’s first focus is developing a drug for dengue fever.
A big part of the reason why we Invested in ILA was because of the people backing the company.
ILA has one of the strongest shareholder registers (major shareholders) we have seen in a while…
Two of ILA’s biggest shareholders and one of ILA’s directors were part of the Race Oncology team that took the company’s share price from ~6.6c to $4 per share.
(a 60x return from a 6.6c cap raise in 2019)
The success of Race Oncology was built on repurposing a drug, just like ILA is planning to do.
Race repurposed drugs for oncology, whereas ILA is repurposing drugs for infectious diseases, where clinical trial success rates are almost 3x higher
After today’s announcement, the team has become even stronger:
- Dr Daniel Tillet - Tillet holds 6.72% of ILA. He was an early backer of Race Oncology (at 6.6c in 2019) and was Chief Scientific Officer when the company’s share price rallied to $4 per share.
- Dr William Garner - Garner holds 14.21% of ILA. He was one of the founders of Race Oncology.
- Chris Ntoumenopolous - Non-executive director of ILA. Chris was also a director of Race during that rally to $4 per share. Chris participated in the most recent ILA capital raise at 15c.
- (NEW) And now, Jason Carrol - Non-executive chairman of ILA who holds ~13.26% of ILA and is also the CEO of $40M capped Tryptamine Therapeutics.
ILA’s recent clinical trial results
A few weeks ago ILA put out preliminary cresults from its Phase 2a/b clinical trials for dengue fever.
The results showed “that ISLA-101 demonstrated clinically meaningful anti-dengue activity, which included a material reduction in viral load and symptoms.”
ILA also reported “meaningful reduction in both viremia (viral load) and symptoms in preventative cohort”
(meaning good for a pill that you take if you are in a high risk dengue location, so IN THE EVENT THAT you get dengue fever it reduces the symptoms - a huge market)
And that “Treatment cohort demonstrated signals of drug effect”
(meaning good early signs for a pill you would take if you have ALREADY CONTRACTED dengue fever, to reduce the symptoms - more info on this expected after ILA has had time to further analyse the results)
The company then ran a webinar to go through the results in detail - which we found extremely useful.

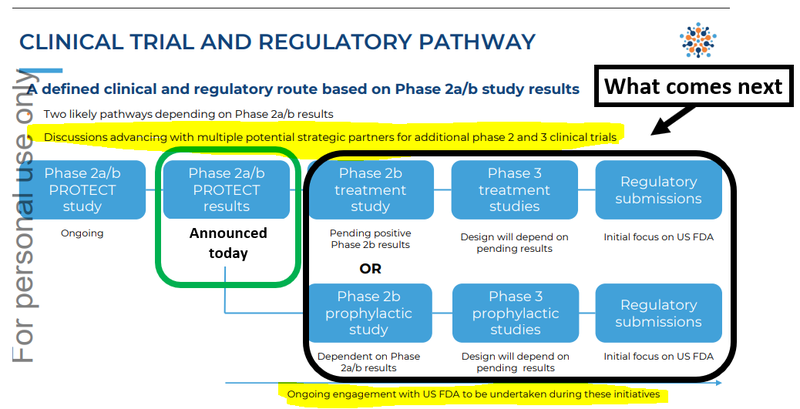
What’s next for ILA?
Phase 2/3 clinical trial design and completion for dengue fever
Now that the phase 2a/2b results are unblinded, we want to see ILA start designing its next trial for dengue fever.
The next stage will be a meeting with the FDA (these generally take about 4-6 months) and then a clinical trial design for the next phase of trials.
Here are the milestones we will be tracking:
🔲 FDA meetings to determine endpoints for the trial
🔲 Clinical trial design completed
🔲 Clinical trial starts
🔲 Clinical trial completed
🔲 Clinical trial results
Galidesivir acquisition
We also want to see ILA make a decision on the option it has to acquire Galidseivir.
ILA is close to securing an exclusive option to acquire a drug called “Galidesivir” that is currently owned by US$2.1BN, NASDAQ listed BioCryst.
Check out our deep dive on Galidseivir here: ILA could also become a biodefense “stockpiling” play
Here are the milestones we are tracking for Galidesivir:
🔲 Option exercise
🔲 Forward plan