EIQ makes FDA submission for its Ai heart failure detection technology
Our AI heart disease detection Investment EchoIQ (ASX: EIQ) just made its FDA submission for its heart failure detection software.
EIQ’s tech is already USA FDA approved AND being used by a growing number of hospitals in the USA to detect one heart disease called Aortic Stenosis.
Now, EIQ is going for approvals for its heart failure detection tech.
And we could get a response from the FDA inside the next two quarters… (Source)
We think FDA approvals for heart failure could be a major catalyst for EIQ because:
- Only 50% of heart failure cases are accurately diagnosed
- 1 in 4 Americans are impacted by heart failure over their lifetime.
- heart failure is the leading cause of rehospitalisation and accounts for 17% of US healthcare expenditure. AND
- The heart failure treatment market is woth ~US$60BN…
EIQ just recently released clinical validation study results for its heart failure tech showing:
- Sensitivity of 99.5% (The ability to identify 99.5% of all patients who actually had heart failure). (source)
- Specificity of 91.0% (the ability to identify 91% of patients who don't have heart failure). (source)
Basically this means that when EIQ’s tech is plugged into cardiologists' workflows, IF the AI says you are positive... 99.5% of the time, the AI is correct...
Which is much better than the only other Ai heart failure detection software used in the market today, which delivers - sensitivity of 87.8% and specificity of 81.9% (source).
The company who developed that software is Ultromics which just raised US$55M in a series C funding round.
According to the source below, the valuation was ~US$300M.
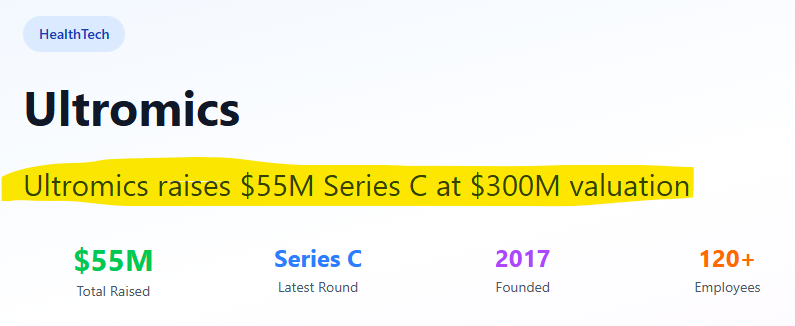
(Source)
EIQ’s data looks stronger AND EIQ’s study was done on a dataset ~13x bigger than Ultromics.
And thanks to Ultormics, a reimbursement code for AI detection of heart failure ALREADY EXISTS.
So IF EIQ can get FDA approval for its heart failure AI model, there already exists a reimbursement code the company can use to commercialise its AI tech.
(reimbursement is how EIQ could get paid every time its tech gets used).
So should EIQ’s AI detection tech gets the all clear from the FDA for heart failure…
… it may be able to quickly start generating revenue using the existing heart failure detection reimbursement code.
Now all we need to see is the FDA clear EIQ’s tech before the market can decide which of the technologies is better (and for the market to value EIQ more appropriately relative to Ultromics).
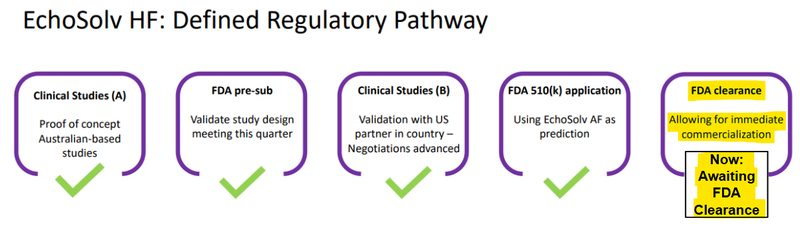
(Source)
And here is a slide from a more recent EIQ presentation expanding on the current step:
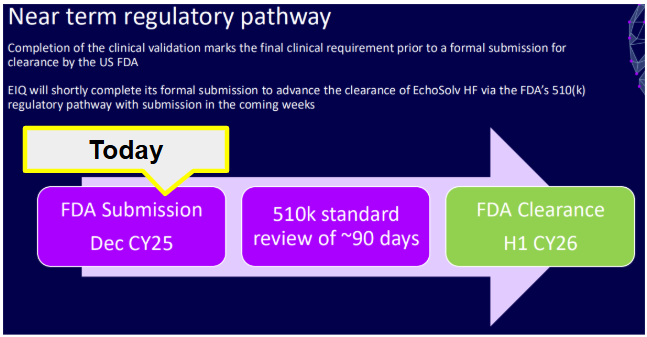
(Source)
So based on this EIQ can expect an outcome in around 90 days, which would be around mid March.
We think the heart failure FDA clearance will be material to EIQ over and above the Aortic Stenosis tech already in the market…
- Because heart failure is a much bigger market to Aortic Stenosis: Healthcare expenditure for AS is ~US$10BN per annum, expenditure for heart failure is ~US$70BN.
- Because the heart failure tech already has an established reimbursement code pathway in place so EIQ can switch on revenues a lot quicker...
What’s next for EIQ?
🔄 heart failure FDA approvals
Next is all about FDA clearance for EIQ’s heart failure detection tech.
As mentioned above, we are expecting to hear back from the FDA inside H1-2026…
🔄 Commercialisation updates for Aortic Stenosis AI tech
The key metric we will be tracking in the short term is how many integrations EIQ can secure for its Aortic Stenosis tech.
In the short term we want to see more distribution deals - either through strategic partnerships or reseller deals.
🔄 Australia and NZ pilot program
EIQ has previously mentioned that this program is being run with a ”leading global structural heart innovation company”.
We want to see some more news on this front because we think it could help advance EIQ’s licensing revenue pathway and be a “proof of concept” study that EIQ can take into the US.
🔄 Partnership with European reseller to broaden market exposure
We want to see EIQ expand into new markets like Europe, in a previous webinar EIQ said the company was pursuing this opportunity and last week the company updated that there was progress on this with negotiations underway.
This also includes CE Mark and TGA applications so that EIQ can sell into Europe and Australia.






