EIQ Investor Webinar - our key takeaways
Yesterday, our AI heart disease detection Investment, EchoIQ (ASX: EIQ) held an investor webinar which we tuned into.
It’s well worth a watch, click the link below to watch a recording EIQ’s latest investor webinar:
Click here to watch a recording of the EIQ investor webinar
The webinar follows the company’s announcement of FDA clearance for its EchoSolv-AS solution and included the introduction of incoming CEO Dustin Haines:

Here our high level key takeaways:
- Dustin Haines brings great experience needed to drive EIQ’s commercialisation strategy
- Good progress being made on reimbursement codes (this is what lets hospitals bill for using EIQ’s tech)
- Strong engagement with Key Opinion Leaders (people who can be ambassadors for EIQ’s tech), AND ongoing negotiations with the Mayo Clinic (perhaps the most prestigious hospital in the US)
- Plenty of newsflow to come
We’ll go through each of these points in more detail below in this Quick Take
First though, the new EIQ CEO…
EIQ’s new CEO Dustin Haines knows how to sell medical advances, he brings experience in roles at mega-caps Gilead Sciences (market cap $173BN) and GSK (market cap $118BN).
At Gilead Science Dustin was VP and General Manager Asia, Middle East, and Turkey.
And at GSK (GlaxoSmithKline) he was a Regional Sales Director.
Welcome to EIQ Dustin.
More on reimbursement code progress for EIQ
Below is the key slide for us which lays out what EIQ has achieved since it received FDA clearance:
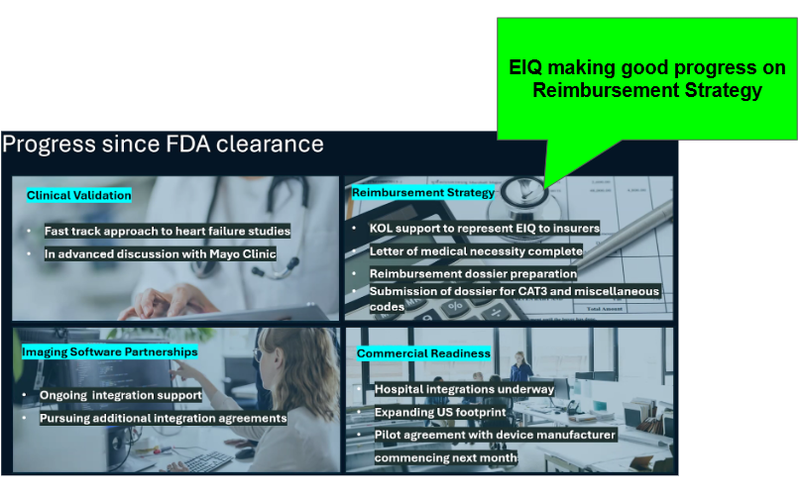
Reimbursement is a payment system where insurance companies or public health programs pay healthcare providers for using EIQ's product.
EIQ receives a percentage of this payment.
This system allows hospitals to use EIQ's technology without passing the cost to patients directly. Reimbursement is crucial for EIQ's revenue generation.
It unlocks commercialization by providing a financial incentive for healthcare providers to adopt EIQ's technology.
To access this revenue stream, EIQ needs FDA clearance and a reimbursement code.
With FDA clearance secured, it was good to see the above progress that EIQ has made on securing reimbursement codes.
The company's strategy involves integrating its technology into as many healthcare providers as possible to maximise potential earnings once reimbursement is secured.
Mayo Clinic negotiations underway
This was news to us - EIQ is negotiating with the Mayo Clinic to get its product used in the hospital:

The Mayo Clinic is a hospital network in the US which has three major campuses in Rochester, Minnesota; Jacksonville, Florida; and Phoenix/Scottsdale, Arizona.
It is widely considered to be one of the best hospital networks in the US, and perhaps the wider world. (Source)
Having this kind of hospital using the tech should only further validate the value of EIQ’s product - and we want to see the Mayo Clinic’s thought leadership drive further uptake of EIQ’s technology.
What’s next for EIQ? Upcoming newsflow
Below is the slide which covered potential newsflow to come from EIQ:

And below is EIQ’s roadmap for it’s Heart Failure product which is the big blue sky opportunity we see in EIQ:
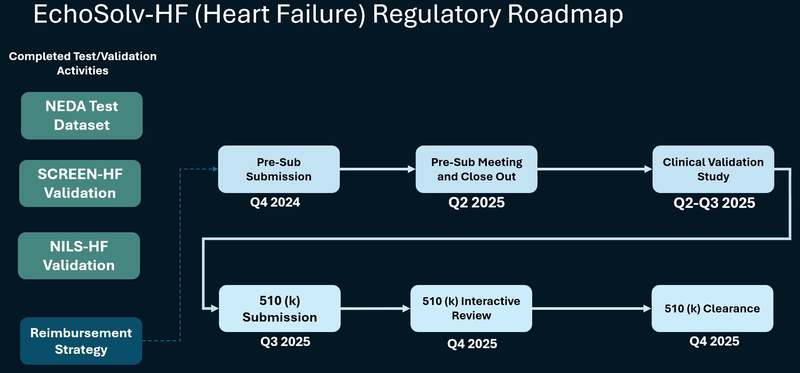
In the short term we’re also looking for progress on this front:
Objective #2: Commercialise aortic stenosis product
We want to see EIQ make sales and grow its revenue for this product.
Milestones
🔄 Reimbursement approvals
🔄 First sales from aortic stenosis product
🔄 Deal with healthcare provider (Pharma company)
🔄 Licensing deal (medical device manufacturer/imaging company)
Source: “What do we expect EIQ to deliver” - EIQ Investment Memo 6 Sept 2024
Read out latest note on EIQ below:

EIQ Secures FDA Clearance - can now market and sell in the USA.






