EIQ - FDA meeting sets out approval pathway for Heart Failure tech
Our AI heart disease detection Investment EchoIQ (ASX: EIQ) just put out an update on its Heart Failure product.
EIQ already has FDA clearance for its AI Aortic Stenosis detection tech (which is now being commercialised).
Next for the company is the much bigger Heart Failure market.
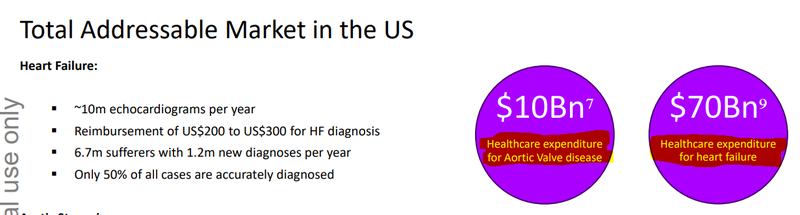
Heart failure is the leading cause of re-hospitalisation in the US, accounting for ~17% of all US healthcare expenditure.
The overall market for heart failure is estimated to be ~US$70BN annually.
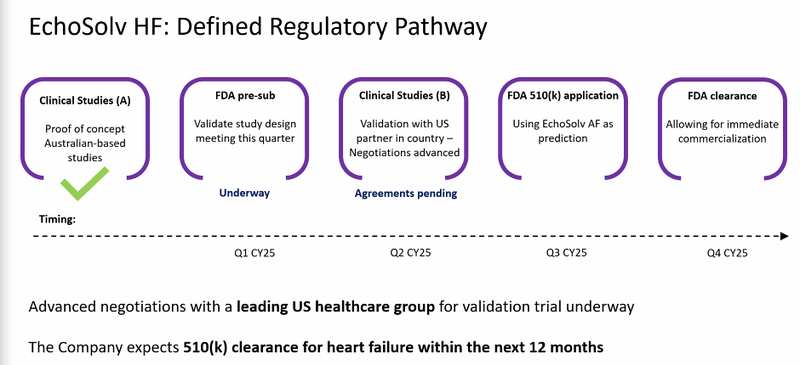
Today, EIQ confirmed that pre-submission meetings had taken place with the FDA for it’s Ai Heart Failure detection tech.
These meetings come ahead of EIQ’s upcoming clinical validation study for Heart Failure.
These meetings are important because it’s EIQ’s chance to engage with the FDA early and understand what they want to see before approving a new piece of tech.
More engagement means better study design which means a higher chance of getting cleared by the FDA.
The Heart Failure validation trials are expected to start this quarter and be completed by mid-year.
EIQ expects to be lodging its formal submission to the FDA in H2 this calendar year.
Mayo Clinic to partner with EIQ for the clinical validation study
EIQ also announced today that a “collaboration agreement” had been signed with Mayo Clinic to complete the validation study.
As part of the deal, Mayo Clinic will also have the right to use EIQ’s Heart Failure detection tech across the groups network of ~30 hospitals.
For context - Mayo Clinic is a hospital network in the US which has three major campuses in Rochester, Minnesota; Jacksonville, Florida; and Phoenix/Scottsdale, Arizona.
It is widely considered to be one of the best hospital networks in the US, and perhaps the wider world.
Having this kind of hospital commit to using EIQ’s tech & assist in validation trials is exactly the type of external validation we like to see for Investments like EIQ.
The Heart Failure validation trials are expected to start this quarter and be completed by mid-year.
EIQ expects to be lodging it’s formal submission to the FDA in H2 this calendar year.
EIQ has already managed to show that it’s Heart Failure detection tech can:
- On it’s own detect 86% of heart failure cases (vs 46% detection in standard clinical practice)
- Together with clinical evaluation increase accuracy to ~97% in high-risk individuals.
97% (using EIQ’ Ai + clinicians) sounds a lot better than the 46% accuracy number in standard clinical practice.
We are hoping that data can be followed up in the clinical validation study & that the FDA ultimately clears EIQ’s tech…
Why Heart Failure clearance could lead to step-change growth for EIQ
First of all, the market for EIQ’s Heart Failure tech is multiples the size of it’s Aortic Stenosis detection tech.
The second part of the story is that the Heart Failure detection tech will benefit from all of the commercialisation work that is going into EIQ’s Aortic Stenosis product.
EIQ is currently pushing to integrate it’s tech into as many hospitals as possible.
It is also looking to get reimbursement codes in place so that the process for EIQ getting paid becomes a lot less frictionless.
In a recent webinar, CEO Dustin Haines and CCO Deon Strydom said that EIQ had ~5-6 hospitals in the integrating stage and ~60 in the pre-integration stage (As well as hundreds in active discussions).
As integrations increase, the future revenue generation potential for it’s Aortic Stenosis product increase.
BUT it also means that when/IF EIQ get FDA clearance for Heart Failure, they don't need to do all of those integrations again.
Instead they benefit from the integration network and can switch on distribution immediately.
Hear Failure also has reimbursement codes in place which will mean EIQ can turn on the revenue potential of it’s product almost immediately…
We wrote about the potential impact of the Heart Failure tech in our last EIQ note here: EIQ - who are they working with?
What’s next for EIQ?
🔄 Commercialisation updates for Aortic Stenosis Ai tech - we want to see EIQ integrate it’s Aortic Stenosis tech into more hospitals in the US.
🔄 Strategic partnership updates - we want to see EIQ advance discussions in this area to help rapidly roll out the company’s tech, grow EIQ’s revenue and build market share.
🔄 Australia and NZ pilot program - this program is with a ”leading global structural heart innovation company” - this will advance EIQ’s licensing revenue pathway and be a “proof of concept” study that EIQ can take into the US.
🔄 Heart Failure validation study with US based Group - EIQ expects the study to start this month and be completed by the middle of the year.
🔲 Partnership with European re-seller to broaden market exposure - we want to see EIQ expand into new markets like Europe, in a previous webinar EIQ said the company was pursuing this opportunity.
🔲 CE Mark and TGA applications - this is so that EIQ can sell into Europe and Australia.






