EIQ clinical validation study underway
Our AI heart disease detection Investment EchoIQ (ASX: EIQ) just kicked off clinical validation studies for its Heart Failure technology.
Late last year, EIQ received FDA clearance for its Aortic Stenosis (AS) Ai detection tech meaning it can start distributing and generating revenues from its tech for the first time.
EIQ is currently working on integration partnerships to try and get the AS tech into as many hospitals/clinics as possible.
While EIQ commercialises its AS tech, the company has also been developing Ai detection tech for Heart Failure which has a much bigger addressable market relative to AS.
Heart disease is the leading cause of deaths in adults, and Heart Failure costs the US healthcare system approximately $60 billion annually.
~10x bigger than aortic stenosis for EIQ.
The addressable market for Heart Failure is expected to be ~10x bigger than aortic stenosis for EIQ.
Here are some of the market value calculations EIQ has shared about the two in the past:
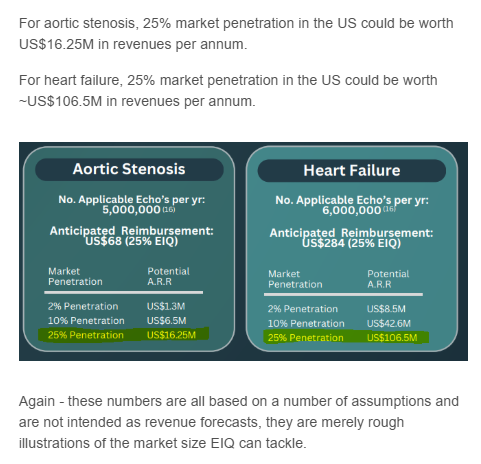
(Source)
Today, EIQ kicked off a clinical validation study for the Heart Failure tech.
The study is a precursor for EIQ’s FDA submission and ultimately FDA clearance which EIQ expects in H2-2025.
FDA clearance for its Heart Failure tech is one of the major catalysts that we think could be a major inflection point for EIQ.
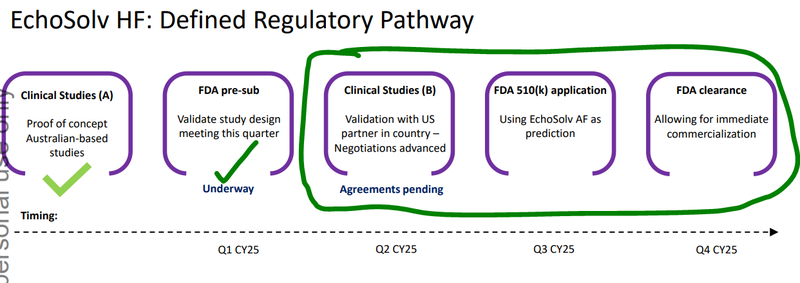
EIQ is already laying the groundwork for distribution of its Heart Failure tech
We mentioned earlier that EIQ is currently focused on integrations for its Aortic Stenosis tech which already has FDA clearance.
At a very high level, integrations are just another way of saying how many cardiologists are committing to using EIQ’s tech in their scanning process’.
The more integrations, means EIQ’s tech is used more often and could eventually lead to more revenues for EIQ…
Integrations are very important because once EIQ’s tech has been put into place in a clinical setting, it means any future technology EIQ’s developed can be rolled out almost instantly…
That’s where EIQ’s Heart Failure detection tech comes into play.
All of the integration work EIQ is doing now means EIQ can instantly roll out HF detection tech to those same networks once the FDA clearance comes in…
This slide from a recent presentation summarised the latest on where EIQ is at with integrations for its Aortic Stenosis tech:
(Watch the full presentation by CEO Dustin Haines here)
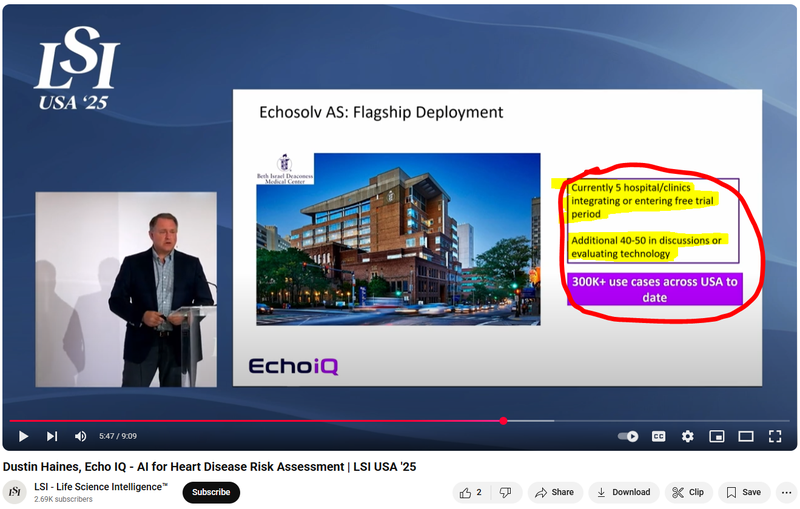
When will revenues come in?
So in the short-medium term, the key metric we are watching is integrations…
But how and when will EIQ start to turn those integrations into revenues?
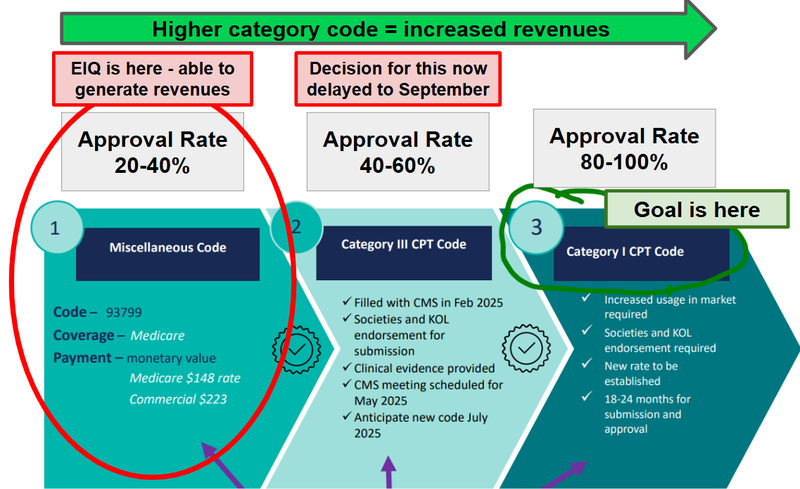
First of all, revenues for Aortic Stenosis products are tied to reimbursement code categories.
The category of the reimbursement code determines how often a user of EIQ’s Aortic Stenosis tech can get paid for use.
EIQ’s tech is currently classified as a miscellaneous code where reimbursement approval rates are ~20-40%.
As the category increases, so do reimbursement rates (Cat III = 40-60% and Cat I = 80-100%).
Right now EIQ’s Aortic Stensosi tech is in the first category…
EIQ was initially aiming to be at Category III by mid-2025 but had a minor setback.
Now that decision is expected to come in September…
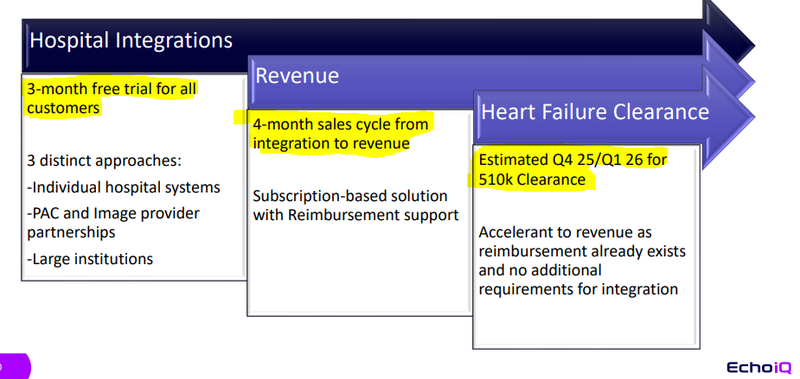
As for the company’s Heart Failure tech… reimbursement process’ are already in place…
So EIQ doesn’t have to go through the same workflows it is doing for its Aortic Stenosis tech.
EIQ also doesn’t have to go through the integration process, because all of that work would have been done while commercialising the Aortic Stenosis product.
So EIQ can just switch on revenues for the Heart Failure tech straight away.
The following slide from EIQ’s recent webinar presentation sums up that transition from integrations into revenues perfectly:
What’s next for EIQ?
🔄 Commercialisation updates for Aortic Stenosis AI tech
The key metric we will be tracking in the short term is how many integrations EIQ can secure for its Aortic Stenosis tech.
In the short term we want to see EIQ’s tech in the hands of more cardiologists around the US.
We also want to see an update on the company’s application to get category III reimbursement codes approved - this is expected sometime in September.
🔄 Strategic partnership updates
We want to see EIQ advance discussions in this area to help rapidly roll out the company’s tech, grow EIQ’s revenue and build market share.
🔄 Australia and NZ pilot program
EIQ has mentioned that this program is being run with a ”leading global structural heart innovation company”.
We want to see some more news on this front because we think it could help advance EIQ’s licensing revenue pathway and be a “proof of concept” study that EIQ can take into the US.
🔄 Heart Failure validation study with US based Group
This is related to today’s announcement.
We want to see EIQ complete these trials and (hopefully) deliver strong enough results to support an FDA submission for its Heart Failure detection tech.
🔲 Partnership with European re-seller to broaden market exposure
We want to see EIQ expand into new markets like Europe, in a previous webinar EIQ said the company was pursuing this opportunity.
This also includes CE Mark and TGA applications so that EIQ can sell into Europe and Australia.






