Broad update from EIQ highlights potential for AI-heart disease detection software
A big update from our AI heart disease detection Investment EchoIQ (ASX: EIQ) today.
EIQ has FDA clearance to integrate and sell its AI-based heart disease detection product into the US.
It is currently at the very early rollout phase, where it is trying to get into as many hospitals and clinics as possible.
Here are the key updates from today:
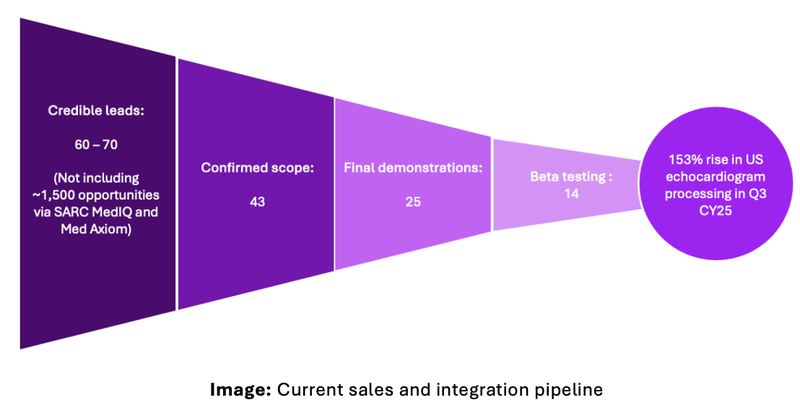
Early traction in US hospitals
What happened: Between July and September 2025 usage of EIQ’s AI detection software increased 153%, with demonstrations and beta testing underway.
Our Take: This is good early traction and uptake of EIQ’s product. There are over 150 leads in EIQ’s pipeline with at least 14 leads in the beta testing phase:
Pilot Trial with “leading global device manufacturer”
What Happened: More than 50,000 echocardiograms have been analysed in 2025 through a pilot trial with a global heart company. This trial is being used to test patient recall and identification of at-risk patients.
Our Take: For device manufacturers, the more at-risk patients identified, the more sales that they can make (generally heart valve replacements or other heart related medical devices). This trial will benchmark EIQ’s product + a cardiologist against just a cardiologist alone. 50,000 echograms indicates that EIQ is well through the pilot trial and we hope that the company moves forward to a commercial agreement with EIQ.
Heart failure study with Mayo Clinic for FDA clearance
What Happened: The clinical validation study on heart failure with the Mayo Clinic Platform is nearing completion. The results will support an FDA 510(k) submission for EchoSolv HF. Clearance would open access to the US$60Bn heart failure market.
Our Take: We see FDA clearance for a second condition (first Aortic Stenosis and next Heart Failure) as a big catalyst for EIQ.
ScImage Partnership Update
What Happened: Echo IQ is progressing site negotiations through its ScImage partnership. Accelerated integrations are expected, leveraging a network of more than 1,200 users across the US.
Our Take: Partnerships and integrations like the one with ScImage are not the ‘sexiest’ updates that a company can provide, but they are crucial to the sales and adoption of EIQ’s product. These technology integrations (particularly in a slow-to-adopt industry like healthcare) can be crucial to unblocking sales.
SARC MedIQ Update
What Happened: Beta testing with SARC MedIQ has been completed and the platform is ready for integration. The reseller network reaches more than 300 healthcare facilities and 1,500 doctors.
Our Take: We see the sales and integration pathway with SARC MedIQ as crucial to EIQ’s ability to penetrate the hospital networks and get its product in the hands of cardiologists. We are hoping for some early traction from the SARC MedIQ relationship… a first hospital deal perhaps?
EIQ hires two new strategic advisors
What Happened: Dr Philippe Genereux, a leading interventional cardiologist, has been appointed as a strategic advisor to support US uptake and regulatory submissions. Dr Asif Ali, a cardiologist and digital health expert, has also joined as an advisor and is advancing opportunities with the University of Texas and Houston Cardiology Consultants.
Our Take: Dr Philippe Genereux is a HUGE get for EIQ. He is one of the world’s top cardiologists and is extremely well connected in the industry. There were new guidelines that he published on Aeortic Stenosis (and now the industry has adopted) where he suggests that asymptomatic patients may need heart support devices (Source). The device manufacturers like this, because it opens up new sales opportunities… but the real kicker is that he is talking about heart valves for moderate risk patients.
EIQ is the only technology that can help cardiologists identify moderate-risk patients for Aortic Stenosis, so we see why this relationship with Philieppe is valueable.
EIQ to expand into the European Union?
What Happened: Echo IQ is in advanced negotiations with a specialist distributor in the EU and plans to apply for CE Mark approval. Europe represents a major opportunity, with heart disease costing €29BN annually.
Our Take: We like it when companies enter new markets, it opens up revenue opportunities and increases the total addressable market for the business.
New Health Economics (NEDA) study underway
What Happened: A health economic study with NEDA is underway to measure the clinical and cost benefits of earlier AI-driven detection of heart disease. This work also supports a collaboration with Harvard’s Beth Israel Deaconess Medical Center. Echo IQ retains exclusive commercialisation rights with NEDA.
Our Take: We see further studies on EIQ’s product as crucial to helping it move up the CMS code chain to better get access to reimbursements from the US Healthcare System.
Overall, these developments demonstrate the growing adoption of EIQ’s AI powered technology and position the company for potential accelerated growth.
The pending FDA clearance for EchoSolv HF could unlock a significant market opportunity in heart failure detection and management.
The company expects that these initiatives will contribute to revenue growth in Q4 this year and will set a platform for a broader scale up in 2026.






