EIQ signs first hospital deal just weeks after successful US FDA approval
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 3,470,000 EIQ Shares and the Company’s staff own 140,000 EIQ shares at the time of publishing this article. The Company has been engaged by EIQ to share our commentary on the progress of our Investment in EIQ over time.
A top US cardiology hospital has just agreed to integrate Echo IQ (ASX:EIQ)’s technology into its practice.
EIQ has an FDA cleared, AI-powered algorithm that helps detect heart diseases.
Yesterday, EIQ announced that it had signed an integration agreement with Beth Israel Deaconess Medical Center in the USA.
(literally just weeks after EIQ successfully obtained FDA clearance)
Beth Israel is a teaching affiliate of Harvard Medical School and ranked in the top 1% of all hospitals in the US for cardiology.
The deal will see EIQ’s AI-based technology used by Beth Israel to help detect heart diseases.
Heart conditions are diagnosed by specialised doctors called cardiologists that read a “scan” of a person’s heart.
These scans are called echocardiograms - or “echos” for short.
The first heart disease EIQ has secured FDA clearance for is called “aortic stenosis”.
Aortic stenosis is a disease where the heart’s aortic valve is narrowed and doesn’t open fully, restricting blood flow to the rest of the body. It can be fatal if left untreated.
1.5 million people in the US alone have aortic stenosis, directly costing the US healthcare system US$10BN (source).
There are currently around 5,000,000 echoes done each year to look for signs of aortic stenosis.
Each year the Beth Israel Deaconess Medical Center (BIDMC) does 30,000 echoes.
Assuming each echo at Beth Israel is done for aortic stenosis, our “back of the napkin” calculation is that this deal could be worth US$510k per year based on a US$68 rebate... this is from just ONE hospital.
(this is a rough calc only, with simplistic assumptions, and is not intended as a revenue forecast - more on this later)
The 30,000 echoes delivered by Beth Israel only represents ~0.6% of the total addressable market of ~5 million echoes per year for this particular condition.
Our key takeaway is that EIQ has now delivered early commercialisation traction just weeks after securing FDA clearance.
This week’s flagship integration agreement with Beth Israel is going to give EIQ’s product significant exposure across the industry.
EIQ says they are expecting further deployments with other hospitals over the coming months...
When trying to sell into slower moving industries like healthcare, having a flagship customer is important to generate momentum in new potential customers.
It provides external validation and “FOMO” for smaller players thinking about adopting EIQ’s product.
In yesterday’s announcement EIQ said that it has also signed up four more hospitals across the US to use its product.
These deals are a direct result of EIQ’s relationship with Core Sound Imaging, a technology partner that plugs into 700 cardiovascular facilities in the US.
On top of that, listening to the recent EIQ investor webinar our ears pricked up when we heard the mention of the “Mayo Clinic”.
The Mayo Clinic is widely considered to be one of the best hospital networks in the US, and perhaps the wider world.
EIQ said that it is in “advanced negotiations” with the Mayo Clinic for clinical validation of its Heart Failure product for use in its hospital network.
(EIQ’s heart failure detection is the next heart disease in its development pipeline to push through FDA clearance)
The two diseases that EIQ has focused on so far are:
- Aortic stenosis: EIQ has secured FDA clearance for its product to be sold in the US. EIQ is now in customer acquisition mode.
- Heart Failure: Leading cause of death among people aged 65+. EIQ is ~ 12 months away from FDA clearance.
EIQ’s revenue model is via reimbursement from insurance companies/healthcare systems every time hospitals use the EIQ technology.
(EIQ and the hospital share the revenue)
Next we want to see:
- More hospitals and partners signed up
- First reimbursements to EIQ (revenue)
- Progress on the heart failure diagnosis towards FDA approval.
In the US ALONE, based on EIQ’s “anticipated reimbursement” numbers net to EIQ (25% of the reimbursement), the aortic stenosis market is worth ~US$85M in revenues per year and ~US$426M for heart failure.
Of course these are very simple back of napkin calcs and are not intended as revenue forecasts - it's more to give a sense of the size of the market at this point in time for EIQ.

(Source)
EIQ doesn't have “customers” in the traditional sense of the word, it is more like partnerships.
EIQ engages with hospitals, but it is the insurance company or public healthcare program that pays for the product.
This is done through a ‘reimbursement code’ that the hospital can use to claim back the cost from the US healthcare system.
EIQ expects to secure a reimbursement code for aortic stenosis “in the coming quarters” - and this is the next big catalyst for the company.
EIQ’s product is predominantly software where margins are usually really high (up around 100%).
The bulk of the costs when developing software happen upfront. Once development is finished, the maintenance costs are usually a nominal amount (hence the high margins).
A software product is also extremely scalable - i.e. you can sell to 100 customers or 10,000 without having to spend a heap of capital.
So once the reimbursement code is secured, the company’s growth is really only limited by the number of hospitals it can partner with.
When it comes to med tech, the market loves high-margin businesses with annual recurring revenues.
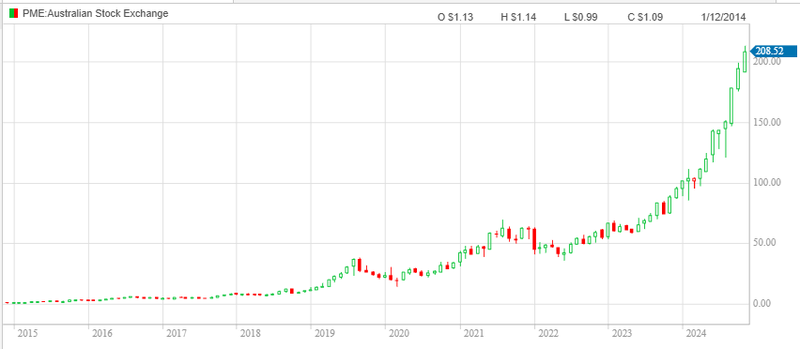
The biggest name on the ASX is Pro Medicus, which is capped at ~A$22BN.
Pro Medicus’ business model is slightly different to EIQ.
EIQ’s tech helps detect heart diseases whereas Pro Medicus’ tech is used by the companies doing the imaging services.
Regardless, we think that Pro Medicus sets the benchmark for how the market values med tech companies that are revenue generating:
Past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
As Pro Medicus grew sales for its technology, its market cap re-rated exponentially.
We are hoping EIQ is re-rated as it grows its revenues.
Of course it's very early days for EIQ and the company is still considered pre-revenue...
EIQ recently hired a new US based CEO to deliver sales revenue and partnerships for the business.
Dustin Haines brings experience in roles at mega-caps Gilead Sciences (market cap $173BN) and GSK (market cap $118BN).
At Gilead Science Dustin was VP and General Manager Asia, Middle East, and Turkey and at GSK (GlaxoSmithKline) he was a Regional Sales Director.
In a recent webinar he articulated the company’s short term goals including optimising for hospital integration and securing reimbursement.

(Check out that webinar in full here)
With FDA clearance secured, a new CEO on board and a newly signed partnership with a top US cardiology hospital, we think that EIQ is well positioned to market its software to new partners over the next few years.
We are hoping yesterday’s announcement acts as a trigger to more commercialisation deals - this forms the basis for our Big Bet which is as follows:
Our EIQ ‘Big Bet’:
“EIQ re-rates to a >$1BN market cap on successful commercialisation of its heart disease detection technology and/or an acquisition at a multiple of our Initial Entry price.
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our EIQ Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
So how much is the deal worth to EIQ?
EIQ’s “reimbursement” model will start generating revenue after the hospital is using the technology.
EIQ can start generating revenues from this deal once reimbursement codes are approved for its tech.
Based on the numbers EIQ put out in its most recent investor presentation, our estimates are that yesterday’s agreement (once revenue generating) could be worth ~US$510K per year for EIQ.
(based on US$68 “anticipated reimbursement) of which EIQ receives 25% multiplied by the 30,000 echoes the hospital performs per year).

(Source)
We expect to see EIQ sign a lot of these types of deals as a way to integrate its technology into the clinical setting and then “flick on a revenue switch” when the reimbursement is secured.
So the hospital will get a fixed amount ($) per echocardiogram through reimbursement and EIQ will take 25% of this fee.
The more echos done, the more money that EIQ makes.
The rebate amount ($) is set by the reimbursement agency.
As shown above EIQ’s “anticipated reimbursement” is for US$68, but it could be higher depending on how EIQ’s product is classified.
It is not entirely clear which reimbursement code will be given to EIQ, but any rebate that is higher than US$68 we see as a potential upside catalyst that will dramatically increase the revenue potential for the company.
Remember each year the Beth Israel Deaconess Medical Center (BIDMC) does 30,000 echoes.
BIDMC is part of a network of 14 hospitals that sit under the Beth Israel Leahy brand - so if EIQ’s product works and there is strong uptake, it should hopefully be rolled out to the full hospital network.
There are currently around 5,000,000 echos done each year for aortic stenosis.
The quantity of Beth Israel echoes only represents ~0.5% of the total addressable market for this particular condition.
EIQ is also targeting heart failure (with a decision on FDA clearance around 12 months away).
For heart failure there are ~6,000,000 echos done each year and the “anticipated reimbursement” that EIQ has quoted before is ~4x higher than Aortic Stenosis (US$284 per echo).
To get an idea of the scale change that could come from EIQ’s heart failure product, below are EIQ’s numbers for the two conditions side by side.
For aortic stenosis, 25% market penetration in the US could be worth US$16.25M in revenues per annum.
For heart failure, 25% market penetration in the US could be worth ~US$106.5M in revenues per annum.
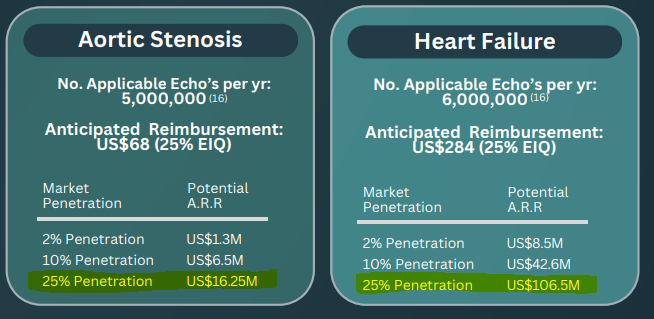
Again - these numbers are all based on a number of assumptions and are not intended as revenue forecasts, they are merely rough illustrations of the market size EIQ can tackle.
If EIQ can commercialise its aortic stenosis product that would make for a really solid business on its own, we see the heart failure product as the blue sky upside potential for EIQ.
Four more hospitals to build traction
In yesterday’s announcement EIQ said that it has also signed up 4 more hospitals to use its product.
These deals are a direct result of EIQ’s relationship with Core Sound Imaging, a technology partner that plugs into 700 cardiovascular facilities in the US.
New EIQ CEO Dustin Haines mentions in his webinar presentation that “IT departments are becoming one of the biggest gatekeepers to integration”.
So deals like the one with Core Sound Imaging make it much easier for smaller hospitals to integrate and use EIQ’s product more seamlessly.
This reduces a big friction point for EIQ to scale.
EIQ mentioned that it is looking at other additional integration agreements and we hope to see other partnerships emerge particularly in different jurisdictions.
Mayo Clinic “name drop” in EIQ presentation
As we pointed out above, listening to the most recent EIQ presentation, our ears pricked up when we heard the mention of the “Mayo Clinic”.
EIQ mentioned that it is in “advanced negotiations” with the Mayo Clinic for clinical validation of its Heart Failure product for use in its hospital network:

The Mayo Clinic is a hospital network in the US which has three major campuses in Rochester, Minnesota; Jacksonville, Florida; and Phoenix/Scottsdale, Arizona.
It is widely considered to be one of the best hospital networks in the US, and perhaps the wider world. (Source)
Having this kind of hospital using the tech should only further validate the value of EIQ’s product - and we want to see the Mayo Clinic’s thought leadership drive further uptake of EIQ’s technology.
How does yesterday’s news affect our Investment Memo?
Yesterday’s news advanced our #2 Objective for EIQ: Commercialise aortic stenosis product.
EIQ now has a partnership in place that it can showcase to new hospitals and build sales momentum going into next year.
We have ticked off this first milestone and added further milestones (in second and third key hospital partnerships) for EIQ to secure:
Objective #2: Commercialise aortic stenosis product
Milestones
🔄 Reimbursement approvals
✅ First partnership from aortic stenosis product
🔲 [NEW] Second partnership with hospital network
🔲 [NEW] Third partnership with hospital network
🔲 Deal with healthcare provider (Pharma company)
🔲 Licensing deal (medical device manufacturer/imaging company)
What's next for EIQ?
In the most recent investor presentation, EIQ announced a number of different short term catalysts that the company expects including:
- Commence Australian pilot program with leading device manufacturer - this will advance EIQ’s licensing revenue pathway and be a “proof of concept” study that EIQ can take into the US. We haven’t talked about this sales strategy much today, but you can read about it here Is FDA approval imminent for EIQ? What happens after that?
- Heart Failure validation study with US based Group - the indication from the most recent webinar is that the US based group is the Mayo Clinic, we think that the outcome of this study will be a big coup for EIQ.
- Partnership with European re-seller to broaden market exposure
- CE Mark and TGA applications (so that EIQ can sell into Europe and Australia).
We are also on the lookout for any news on the reimbursement code or any surprise “sales” into new hospital networks.
EIQ also says that the pre-sub submission to the FDA on its heart failure product is scheduled before the end of this year / early next.
What could go wrong?
At this stage of EIQ’s journey a big risk for the company is sales risk.
With FDA clearance secured and an extremely high-margin product, EIQ’s growth is now directly dependent on its ability to convince hospitals to integrate and use its product.
Sales/Commercialisation risk
EIQ is reliant on its partners, both under the licensing and reimbursement strategy, to use EIQ’s product. If EIQ’s product is not used (because it doesn’t add value back to the provider), then it won’t be able to generate revenue. There is no guarantee that EIQ’s product will be used by its partners and, therefore, no guarantee of revenue.
Source: “What could go wrong” - EIQ Investment Memo 6 Sept 2024
There is also an element of “regulatory risk” as well.
From a revenue perspective, the reimbursement code assigned to EIQ’s software will determine how much the company makes each time its software is used.
EIQ is working with reimbursement specialists to provide the best application possible to the insurance providers, but there is no guarantee.
A reimbursement of $50 or $68 or $284 could make a BIG difference to the company’s revenue potential. We would expect these figures to become clearer over the coming months as EIQ progresses this work.
Regulatory Risk
EIQ has applied for FDA clearance under the 510(k) pathway for aortic stenosis and will apply for FDA clearance for heart failure.
There is no guarantee that the FDA will provide clearance, and the application may be rejected.
Also, EIQ’s strategy is reliant on securing reimbursement for its product.
If EIQ is not able to secure a reimbursement deal then its commercialisation strategy may need to pivot.
Source: “What could go wrong” - EIQ Investment Memo 6 Sept 2024
Our EIQ Investment Memo:
In our EIQ Investment Memo, you can find the following:
- What does EIQ do?
- The macro theme for EIQ
- Our EIQ Big Bet
- What we want to see EIQ achieve
- Why we are Invested in EIQ
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

