TRI announces positive engagement with FDA
Today our AI mental health Investment TrivarX (ASX: TRI) announced a positive engagement with the US FDA following a pre-submission meeting for its algorithm to detect a current Major Depressive Episode (cMDE).
The algorithm is called MEB-001.
The positive engagement helped TRI gain confidence in their clinical trial design for the planned pivotal study of MEB-001:
- The upcoming pivotal trial will involve at least 563 patients across at least 5 US study sites
- TrivarX is in advanced discussions with prominent US sleep centers and hospitals to secure high-volume trial locations
Successful completion of this pivotal study is the last step TRI needs to complete before submitting MEB-001 for FDA approval via the De Novo pathway.
MEB-001 was originally trained on sleep study data collected in a sleep clinic (which looks like this for patients):
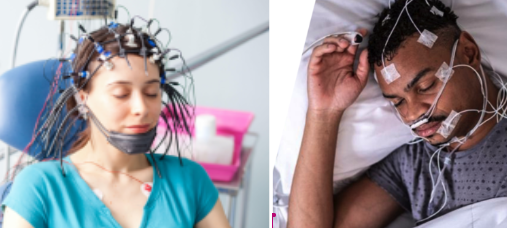
But the big blue sky upside for us is TRI’s ability to move into wearable technology:

Previously in November TRI announced success in the first step for screening for mental health disorders using ‘wearable’ technology.
We are talking about devices like Fitbits, Apple Watches or Oura rings.
This opens up the potential to screen for mental health issues at home while you sleep wearing your smartwatch.
TRI is working to make it possible.
We wrote about TRI’s algorithms emerging potential application to wearables in our latest TRI note:

TRI announces smartwatch and wearables screening for mental health issues.
And below is an image which summarises the role of TRI in this rapidly emerging field of mental health:
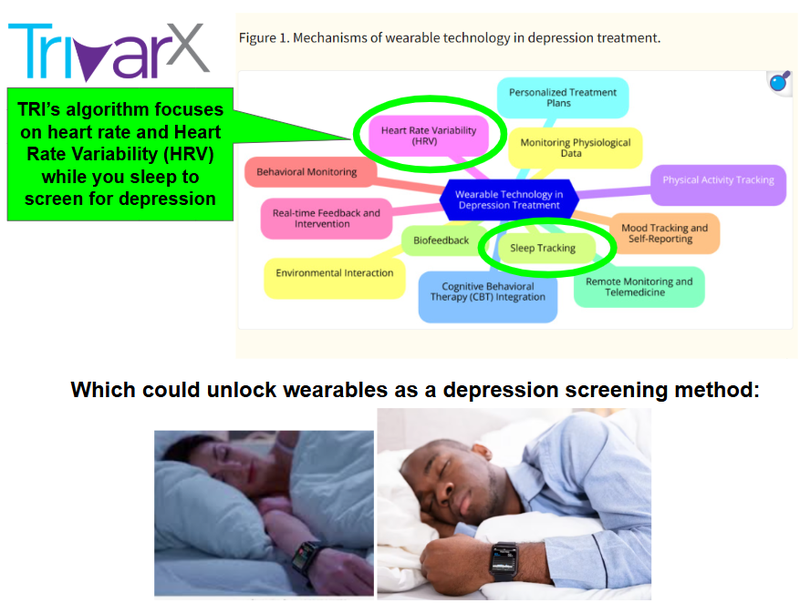
(Source)
More research in this field comes out every month.
This recent piece of research in Psychiatry Research below highlights the growing and potentially very important role of wearable devices in enhancing depression treatment by monitoring real-time physiological data:
(Source)
Actigraphy is a non-invasive method that uses wearable devices to measure movement and activity patterns, providing insights into sleep-wake cycles and overall physical activity.
It is commonly used in sleep studies and behavioral research to assess conditions like insomnia and circadian rhythm disorders.
This study explored the use of wrist-worn actigraphy data and machine learning (ML) techniques to detect Major Depressive Disorder (MDD) using the Patient Health Questionnaire-9 (PHQ-9).
Results showed movement-related features and nighttime movement as key indicators for detecting MDD.
This is exactly what TRI Chief Medical Officer Dr. Archie Defillo says in his presentations that he believes “Sleep is the window into mental health”.
Bear in mind, that this research was published in February of last year, just before TRI released positive clinical trial results for its algorithm:

TRI’s Phase 2 Clinical Trial Results are in…
What about other mental health conditions beyond depression?
So can TRI do it for other mental health issues like anxiety, PTSD, bipolar, Alzheimer's, etc?
(all huge markets)
Quite possibly, we think it could look like this:
- Use Artificial Intelligence on detailed sleep data (biometric data, EEG and ECG recordings) from sleep clinics (EEG requires all those head electrodes) to identify the specific brain signals correlated to a particular mental health disorder.
- Prove it's accurate with clinical trials.
- Develop AI to identify correlation to the same signal in the less detailed, but widely used ECG wearables data.
- Add the newly screenable mental health condition to the growing portfolio of mental health screenings.
Repeat steps 1 to 4 for as many mental health conditions as possible.
Then, ideally be acquired by one of the wearable makers - think Apple, Google, Fitbit or an up and coming smartwatch/wearable company.
TRI is still at the early stages and there is a lot of work to do.
But another bit of recent research from May last year in the highly prestigious journal eBioMedicine (part of The Lancet family) suggests that wearable technology could also be applied to bipolar disorder as well (using a FitBit no less):

(Source)
This study in Korea used sleep onset and offset data collected through the Fitbit Charge device to estimate sleep and circadian phases.
In turn aiding in possible early interventions:
“Integration of wearable devices and mathematical models can detect disruptions in circadian rhythms in real time.This opens up the possibility of assessing the risk of recurring mood episodes in the patient's daily life outside clinical settings. Additionally, it [a wearable like a FitBit] provides an objective measure of the patient's condition, which has been lacking in traditional interview-based psychiatric approaches to mood disorders. Based on these findings, clinicians may be able to intervene early, before daily mood swings develop into full-blown mood episodes.”
While we await further progress from TRI on wearables, we are now looking forward to the pivotal study.
What’s next for TRI?
We now want to see TRI progress through the following milestones to complete a pivotal study.
This will allow TRI to submit its algorithm to FDA for approval via the De Novo pathway.
Objective #4: Complete Pivotal Study
Milestones
🔲 Complete Pivotal Study Design
🔲 Appoint CRO for Pivotal Study
🔲 IRB Approval for Pivotal Study
🔲 Commence Pivotal Trial
🔲 Interim Results
🔲 Complete Recruitment
🔲 Final Results






