NTI lays out partnership & registration strategy
Our biotech Investment Neurotech International (ASX: NTI) just put out an update on its partnering/registration strategy for its NTI 164 treatment.
The company’s NTI 164 treatment is primarily focused on paediatric neurological disorders.
Over the past 12 months NTI has put out clinical trial results on three different disorders:
- Phase 1/2 for PANDAS/PANS
- Phase 2/3 for Autism Spectrum Disorder (ASD)
- Phase 1/2 for Rett Syndrome
We think the data from all three of those trials have been solid, especially when compared with data for existing treatments of those disorders…
And especially in the ASD case, where there are no current approved treatments…
With all of that data now in hand, NTI has now laid out what the company’s strategy is going forward.
Here were some of our key takeaways from today’s announcement:
1. NTI looking for “one or more” strategic partnerships
NTI explicitly said it will look for “one or more strategic partnerships for NTI164 in the United States
(US), Europe and certain Asian territories (e.g. Japan)”.
We think strategic partnerships are major share price catalysts for biotech stocks.
Oftentimes, despite solid clinical trial data, biotech investors will look for external validation of a company’s progress before deciding whether to buy into a company or not.
External validation like - FDA approvals (regulatory validation) or from deals signed with big pharma companies (industry validation).
A recent example is the deal our other biotech Investment DXB deal worth up to $230M.
Dimerix’s share price rallied from its 2023 lows to a high of over 40c off the back of that news.
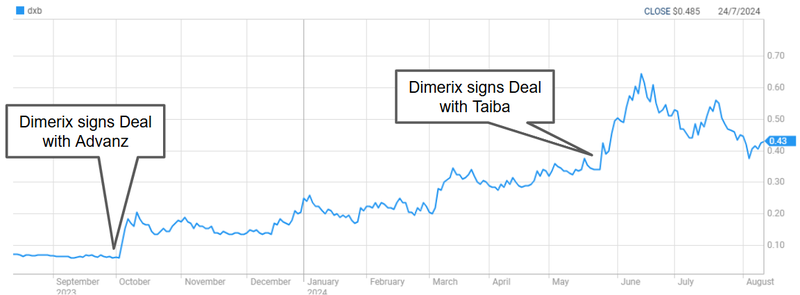
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
Strategic partners are all huge for small cap biotechs because they bring in the financial firepower to fund the later stage development costs for treatments.
NTI explicitly said it would look at “licensing transactions, equity-based investment(s) or M&A” - we think any of these could be big for NTI.
The slide from todays announcement tabling interesting the total addressable market sizes for the disorders NTI is targeting in Australia gives good context on any deals that might happen:
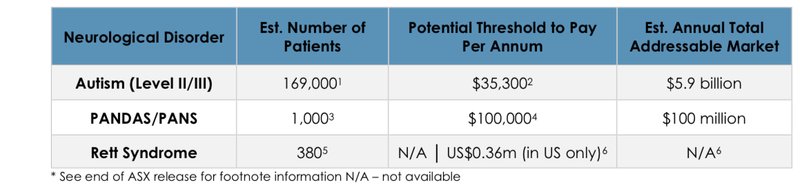
2. Orphan Drug Designation (ODD) applications lodged
NTI confirmed that it recently “lodged its first Orphan Drug Designation (ODD) request for PANDAS/PANS with
the US Food and Drug Administration (FDA)”.
NTI also said it was planning ODD filings for Rett Syndrome in the US and PANDAS/PANS in Europe.
ODD for small cap companies can be very important because of the exclusivity and accelerated approvals process for a treatment.
Lear more about what makes Orphan Drug Designation important here:

3. Pre-Clinical Toxicology and Human Pharmacokinetic (PK) Studies to be completed Q1 2025
These are precursor requirements for all regulatory filings.
NTI said these would be complete “before the end of Q1 CY2025”.
4. Provisional registration in Australia (TGA)
NTI is planning TGA meetings for its PANDAS/PANS or Rett Syndrome treatments by Q2 2025
Submissions for approval are expected to then be lodged in H2 2025 - with the registration process taking ~ 220 days to complete that will mean this newsflow comes in 2026.
What’s next for NTI?
- FDA response on Orphan Drug Designation expected in ~3 months
- Advancing NTI164 through clinical pipeline across multiple rare neurological disorder indications (including Cerebral Palsy)
For a full rundown of what we want to see next from NTI, read our latest note:

NTI’s trial of Autism treatment in children shows further patient improvement after 12 weeks






