EIQ - our key takeaways from the EIQ webinar yesterday:
Our AI heart disease detection Investment EchoIQ (ASX: EIQ) hosted an investor webinar yesterday morning.
For those who missed it, here is a recording to that webinar:

(Source)
We were on the call and there were a few fairly interesting points that were discussed which we thought would be worth highlighting.
Here were our key takeaways from yesterday's webinar:
- CEO Dustin gave a pretty solid visual overview of how EIQ’s tech works - He talks over slide 9 in the webinar, for anyone new to EIQ. it’s a pretty detailed (but short) overview of EIQ’s tech and how it works.
- On the accuracy of EIQ’s tech for Aortic Stenosis - (Slide 10 & 11) Dustin talks about the misdiagnosis/underdiagnosis issue for heart problems and how EIQ’s tech, combined with a cardiologist helps increase that diagnostic accuracy to ~98.6%. Interestingly, EIQ’s tech is able to detect mild/moderate (asymptomatic) aortic stenosis, which he said mostly goes undiagnosed.
- Total Addressable Market for aortic Stenosis - Dustin talked about how Aortic Stenosis is costing the US healthcare system ~US$10BN a year and only 60% of cases are being accurately diagnosed.
- Progress made over the last 60 days - Dustin then covered slide 15 of the presentation. This one was really good, he talked about how ~60 clinics/hospitals are in discussions to integrate EIQ’s tech into their business’. He also talked about how the sales team had been trained and briefed in Jan and were “out in the field” in Feb. The main signal we got from Dustin was that right now, EIQ is going pretty hard on building up as big of a “lead” of potential hospital’s/clinics as possible.
- 2025 commercial pathway - Dustin broke down the sales cycle for EIQ - 4 months from first trials being started to sales revenues starting. EIQ currently have “5-6 integrating” and “roughly 60” at that pre-integration stage. He also said “hundred’s” were in discussion. Guidance was for sales to really start ramping up in the second half of this year.
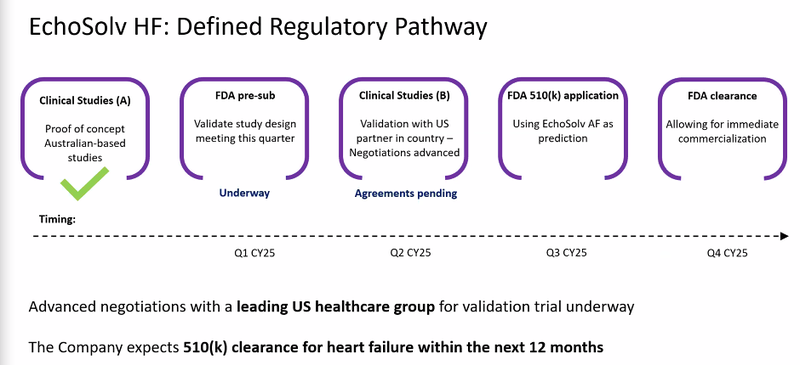
- Heart Failure FDA clearance to be a game changer - Dustin’s commentary on the upside of it’s Heart Failure tech was the highlight of the webinar for us. He mentioned that all of the integration and sales work that EIQ would be doing for Aortic Stenosis would mean EIQ dont have to go and do all that work again for Heart failure.
- Heart Failure FDA Clearance in Q4-2025/Q1-2026 - EIQ’s Heart Failure FDA clearance is targeted for the end of the year. Current practices mean ~50% of Heart Failure is misdiagnosed/underdiagnosed. EIQ by itself picks up 86%, EIQ + a cardiologist picks up 97%.
- On US reimbursement strategy for Aortic Stenosis - Three different phases and eIQ is anticipating a new code in July. Then expectation is ~18-24 months for submission/approval of Cat 1. Category matters because it improves reimbursement approval rates (Misc code = 20-40% approval, cat 3 = 40-60% approval, Cat 1 = 80-100% approval)
- EIQ going after Subscription model - looking to charge customers a fee that allows for hospitals to play a subscription fee and give the hospital the opportunity to make money above what they pay EIQ if they can get higher than average approvals for reimbursement. Subscription model scales as the category for reimbursement increases.
CEO Dustin Haines and CCO Deon Strydom also did some Q&A at the end of the call which is also worth sticking around for.
What’s next for EIQ?
🔄 Strategic partnership updates - we want to see EIQ advance discussions in this area to help rapidly roll out the company’s tech, grow EIQ’s revenue and build market share.
🔄 Australia and NZ pilot program - this program is with a ”leading global structural heart innovation company” - this will advance EIQ’s licensing revenue pathway and be a “proof of concept” study that EIQ can take into the US. We haven’t talked about this sales strategy much today, but you can read about it here Is FDA approval imminent for EIQ? What happens after that?
🔄 Heart Failure validation study with US based Group - the indication from EIQ’s most recent webinar is that the US based group is the prestigious Mayo Clinic, we think that the outcome of this study could be a big coup for EIQ.
🔲 Partnership with European re-seller to broaden market exposure - we want to see EIQ expand into new markets like Europe, in a previous webinar EIQ said the company was pursuing this opportunity.
🔲 CE Mark and TGA applications - this is so that EIQ can sell into Europe and Australia.






