Dimerix reveals “material offers received from multiple parties"
Our Phase 3 biotech Investment, Dimerix (ASX:DXB), released a new investor presentation today, which revealed partnership negotiations with, “material offers received from multiple parties”.
DXB is getting mighty close to a key catalyst later this year, which could unlock a significant re-rate if the results are positive.
These results could underpin the value of DXB’s potential partnership deal(s), as it looks to provide a safe, effective treatment for a nasty kidney disease called FSGS.
Here are our key takeaways from the presentation:
- “Advanced partnering negotiations with material offers received from multiple parties for various territories” - DXB has revealed that the partnering process is well underway and that’s great news for us as DXB Investors. We’d long suspected that this was happening in the background, but to have it confirmed was a major plus. In our latest note, we took a look at what drug pricing and partnership deal size could look like.
- Interim analysis due “end 2023” - we were hoping for a Q3 result here, but it could stretch into Q4 now. While we are eager for these results, there could be benefits to this, with the results potentially coming during more favourable market conditions for biotechs.
- 21% market growth year on year - we think the expanding market for FSGS could improve any deal terms that DXB is able to secure.
In addition to today’s presentation, we note a recent research paper on FSGS which can be accessed here.
It’s a big read, and we aren’t nephrology experts - but we find the paper has a lot to say on something called monocyte chemoattractant protein-1 (MCP-1).
MCP-1 is a key part of the inflammatory pathway in FSGS.
Previously in Phase 2, DXB was able to observe a 39% reduction in MCP-1 in the urine of FSGS patients treated with DMX-200 compared to placebo.
Short version: we think new independent research points to DMX-200 (DXB’s drug) targeting a critical part of how patients' FSGS gets worse - which could increase the likelihood that the interim analysis results are positive.
Remember as well, DXB has orphan drug status - meaning there are a range of incentives available to it from regulators to speed its entry into the market.
In the context of orphan disease treatments, we also really liked this slide which showed the rapid share price appreciation Neuren Pharmaceuticals experienced after a long dormant period, following the release of top line Phase 3 data:
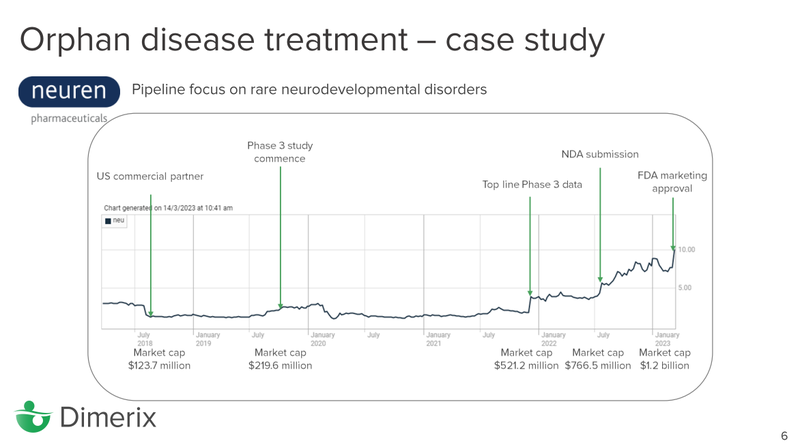
While there are no guarantees that DXB will follow a similar trajectory - and a lot rests on the interim analysis result - we think the Neuren orphan drug story has strong parallels to the DXB story.
Today’s slide deck will be presented both online and in person on Friday at Emergence23 Wholesale Investor conference in Sydney.
To register for the conference follow the link below:
https://www.emergence.wholesaleinvestor.com/attend-sydney
What’s next for DXB? We’re looking for additional patient recruitment updates on the 144 patients DXB needs to complete Part 2 of the trial which could unlock accelerated marketing approval.






