Dimerix clears an important hurdle
This morning our biotech Investment, Dimerix (ASX:DXB), announced some positive news - the Data Safety Monitoring Board (DSMB) recommended that the company’s Phase 3 FSGS trial continues.
The immediate takeaway from today’s milestone is that it adds further evidence to support the safety profile of DXB’s DMX-200 drug and it means DXB is on track to deliver on a key catalyst - the Part 1 interim analysis which is due in the second half of this year.
It also satisfies the bull case we laid out in our latest DXB article:
- Bull Case: Enough evidence to continue the trial
- Bear Case: Trial paused or stopped
The DSMB has the following functions:
- safeguarding the interests of study participants
- ensuring that definitive and valid results are produced which will reliably inform the future treatment of patients
- enhancing the credibility of the trial.
Importantly, the DSMB has access to unblinded data - meaning it knows how DXB’s treatment is tracking.
While we had been expecting this news, it's good to have the added certainty and this result de-risks the trial as DXB approaches a critical period.
The chart below shows the chronology of DXB’s Phase 3 trial:
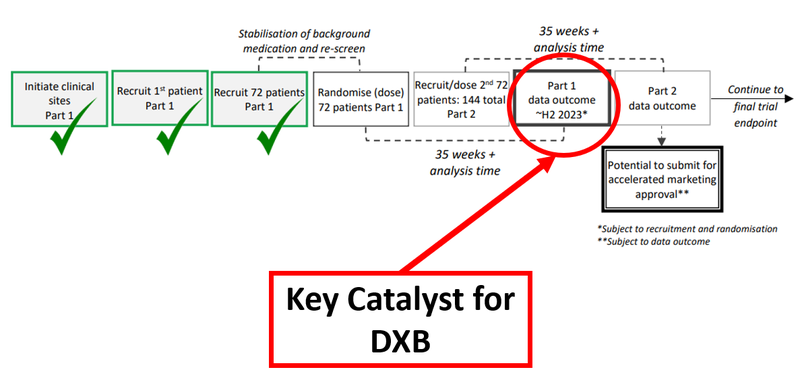
This Phase 3 trial is for DXB’s treatment for FSGS, a rare kidney disease that often results in expensive dialysis. There are no existing approved treatment options for those suffering from it - some 220,000 people have FSGS in 7 major markets. The market for FSGS treatments is estimated to be worth US$12.6BN due in part to premium orphan drug pricing.
Today’s update included the news that DXB has 96 enrolled patients. A final total of 144 is needed for the Part 2 data outcome, which would unlock the opportunity for DXB to apply for accelerated marketing approval.
The patient recruitment has been lumpy - but the overall trend is remarkably quick, which we think underlines the speed and efficiency with which DXB has rolled out this Phase 3 trial.
Our anticipation is building ahead of the Part 1 interim analysis — we suspect that behind the scenes competitive tension could be building around a potential partnership for DXB.
We note that Travere Therapeutics, which is working on a complementary FSGS treatment to DXB, has secured a long term licensing deal with Vifor Pharma with a value of up to $845M.
Vifor, in turn, was acquired by ASX biotech giant CSL for $18.8BN due to its R&D pipeline of renal treatments undergoing clinical trials.
Meanwhile, despite the currently out of favour nature of the biotech sector, famous investor Chamath Palihapitiya’s special purpose acquisition company (SPAC) put together a total funding package of US$825M via a merger for ProKidney (now valued at US$2.5BN) in January to advance Phase 3 development of a cell therapy for kidneys:

We think this points to a lot bubbling beneath the surface for biotechs that are targeting the kidney (like DXB) and is a major reason why we are eagerly looking forward to DXB’s Part 1 interim analysis results.






