TRI’s Phase 2 Clinical Trial Results are in…
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 18,820,000 TRI shares at the time of publishing this article. The Company has been engaged by TRI to share our commentary on the progress of our Investment in TRI over time.
Our AI mental health Investment, Trivarx (ASX:TRI) just published the results of the much anticipated Phase 2 clinical trial.
TRI’s technology analyses a patient’s sleep data and screens for mental health conditions, and the company is currently focussed on depression.
Today’s TRI phase 2 results on 400 patients continued to deliver the “screening for depression” accuracy better than or inline with the much smaller patient numbers in Phase 1.
So it is now confirmed that the “better than current standard of care” results from TRI’s successful Phase 1 trial results were not just a statistical “fluke”.
TRI’s results are repeatable across a much larger group of patients.
So the next step now is for TRI to do a final “pivotal study” to submit for FDA approval and then get their mental health screening tool into the market.
TRI’s Chief Operating Officer Kai Sun presented at the TechKnow conference in Sydney this morning:
Click here to watch TRI’s presentation on these results from this morning
And click here to view the TRI presentation slides
Beyond the next study, we also want to see some progress on TRI’s “screening for mental health issues” tech moving into wearables like Fitbit or Apple Watch.
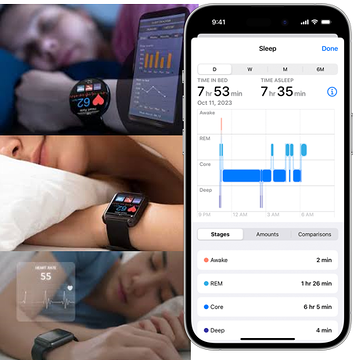
Today’s Phase II clinical trial results confirmed that TRI’s AI driven algorithm to detect current Major Depressive Episodes is competitive with the current standard of care - which is a crude, basic survey you conduct by yourself with a pencil and paper
You may have taken one of these surveys before, and struggled to ‘rate’ yourself:
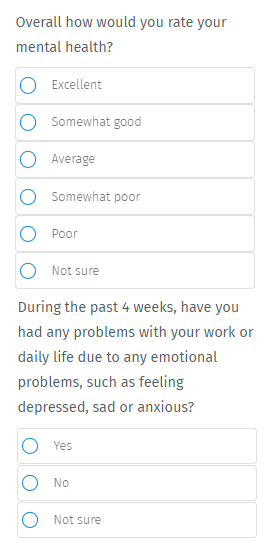
The current way of doing things is hardly “revolutionary” in this age of fancy wearables and data collection.
TRI’s AI technology screens for mental health issues like depression by analysing a patient’s sleep data.
It has just completed a Phase 2 study on 400 patients with the goal of showing that its tool is effective at screening for a current Major Depressive Episode (cMDE).
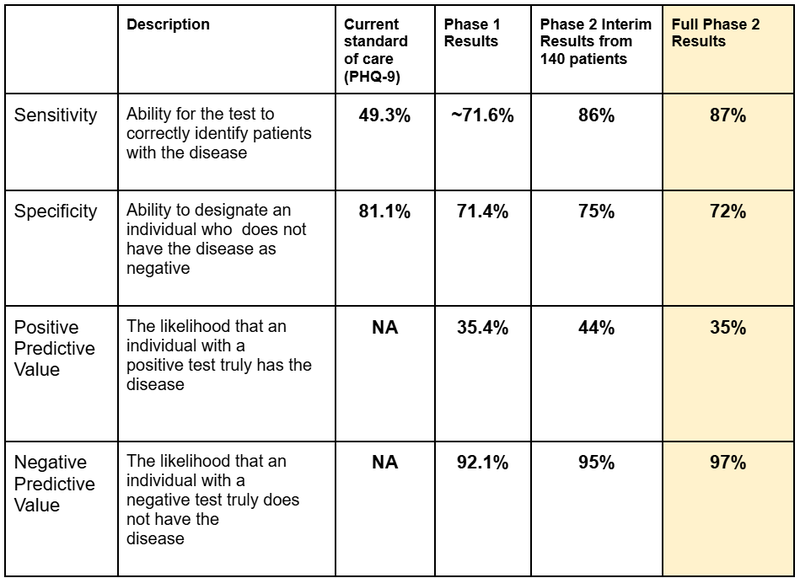
The results showed that compared to the Phase 1 trial, “Sensitivity” increased from 71.6% to 87%.
Sensitivity is the measure of the ability of the algorithm to identify patients with the disease.
The current standard of care has a less than 50% ability to correctly identify a patient with the condition.
So 50% of the time the current standard of care gets it wrong...
TRI’s algorithm had an 87% chance to identify a patient with the condition.
Here is how the headline results stacked up:
Ultimately, TRI’s results continue to demonstrate a big improvement on the current standard of care.
It’s also a large improvement on the company’s Phase 1 results and in line with what was known from the interim results published in February, fitting into our ‘Base Case’ expectations for these trial results.
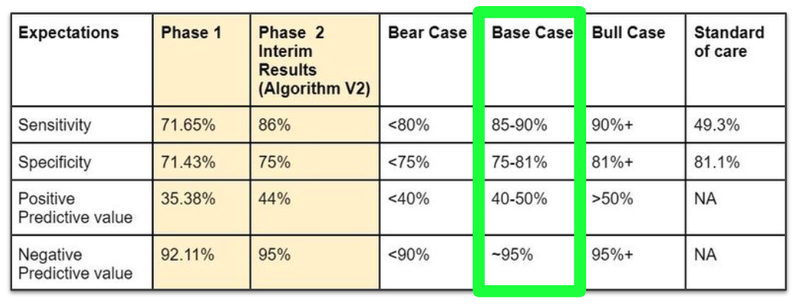
(Source - Next Investors, 10 July)
Confirming the final Phase II results from the interim trial is an important de-risking factor for the study.
The interim results published in February only used a small sample size of 140 patients. These final results were from 400 patients and confirmed that TRI’s algorithm is effective at screening for current Major Depressive Episodes.
TRI has indicated in its most recent presentation that this data provides it with enough information to build algorithms to diagnose for other conditions through sleep:
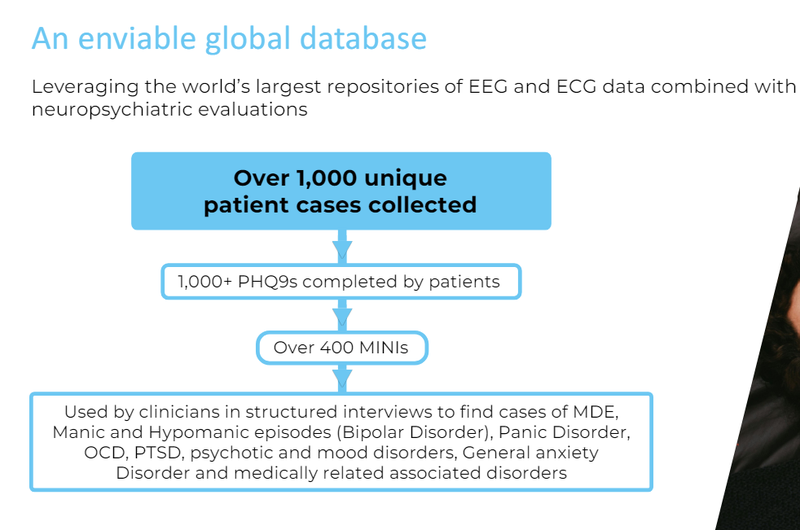
(Source)
Next, we expect TRI to follow up this study with a final “Pivotal Study” that is expected in December this year.
This final study should set the stage for FDA approval for the company in the second half of 2025.
TRI brings on Resapp founder - Resapp sold to Pfizer for $180M
Notably, TRI recently added Dr. Tony Keating to the board:

(Source)
Dr. Keating brings a wealth of experience in securing approval from the FDA for algorithm based products.
Most importantly, as a co-founder of Resapp, Dr Keating has ‘been there and done that’ with an ASX listed biotech and been part of a team that delivered a successful exit to Pfzier.
ResApp is the ASX-listed app-tech story that TRI is looking to follow.
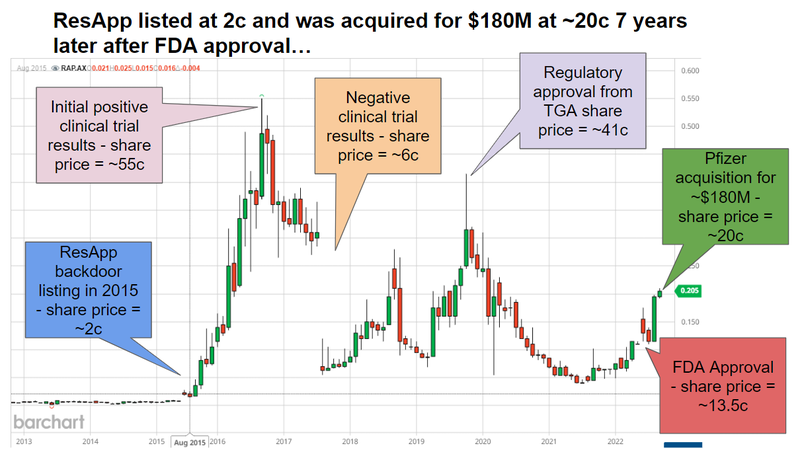
Roughly 10 years ago ResApp developed a diagnostic tool that could detect and identify various respiratory conditions based on the sound of a cough made into a smartphone.
ResApp began at a University of Queensland start up and did a backdoor listing in 2015 at a market cap of just ~$11M and at ~2c.
In 2022, seven years after listing, Pfizer acquired ResApp for ~$180M at a share price of 20.8c:
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
ResApp went through a couple waves of strong sentiment, before it was ultimately acquired at a nearly 1,000% share price premium to the entry price for investors that backed the company when it backdoor listed and held all the way through.
We are Invested in TRI with the hope that it can deliver a similar outcome.
There’s no guarantee of success of course - this is early stage, high risk investing.
One of the key challenges that ResApp faced was securing the FDA approval.
FDA approval allows a company to sell a product or service in the US market.
The company once had its approval knocked back - before it was eventually secured in advance of the Pfizer acquisition.
We think that Dr. Keating’s experience dealing with the FDA, in particular having a successful track record of securing approval for algorithm based technology is a big plus for TRI.
We are Investing in TRI to hopefully see it achieve similar success by first validating its technology in clinical trials, getting regulatory approvals and then eventually commercialising the tech.
This brings us to our TRI Big Bet...
Our TRI Big Bet:
“TRI re-rates to a $250M plus market cap on successful clinical trial results for screening one or more mental health disorders, FDA approval and/or is acquired for multiples of our Initial Entry Price”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our TRI Investment memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true. NOTE: FDA approval added to big bet 10 July 2024.
More on today’s clinical trial results.
Today, TRI published the results of its Phase 2 study.
Key highlights include:
- Significant improvement in “Sensitivity” data: from 71.6% in Phase 1 to 87% in Phase 2.
- Significant improvement in Negative Predictive Value: 92.1% in Phase 1 to 97% in Phase 2
- Consistent Specificity and Positive Predictive Value from Phase 1.
These results fit into our “Base Case” scenario for the company as they are in line with the interim results published in February earlier this year.
We think this data sets up TRI with enough information to go and conduct a larger “Pivotal Study” that will set the stage for FDA approval of the treatment.
Also, this data shows that TRI’s tech is effective at identifying mental health conditions like current Major Depressive Episodes...
which backs up TRI’s Chief Medical Officer Dr. Archie Defillo comments when he says “sleep is the window into a person’s mental health”.
Therefore, we expect TRI to go on and use what it has learned here to develop sleep diagnosis tools for other mental health conditions.
Patients in TRI’s trial were monitored for sleep patterns in a physical location called a sleep clinic.
TRI’s algorithm evaluated the sleep data from these patients and identified patterns of brain waves (EEG), an electrocardiogram (ECG) and heart rate variability (HRV) to identify signs of depression.
So there are 3 variables that TRI is looking at - incidentally this is where the TrivarX’s name comes from - Tri (three) Var (iables) X (what are we looking for?).
So far what TRI has shown it is very good at screening for is current Major Depressive Episodes...
And with vast volumes of data and a powerful algorithm, TRI may be on the cusp of providing an added layer of objectivity in the somewhat subjective world of mental health screening.
(it is quite sci-fi stuff, but if you want to TRI Chief Medical Officer Dr Archie Defillo talk more about the science and technology watch this excellent video)
In close proximity to collecting the sleep data, the patient also undertook a Mini-International Neuropsychiatric Interview (also known as a MINI).
This is a well accepted way of diagnosing clinical depression.
Screening is different to diagnosing - “screening tests are primarily used for early detection of disease or risk factors whereas diagnostic tests are used to [formally] establish the presence or absence of disease.” (Source)
TRI’s screening is then evaluated against the results of the MINI (diagnosing) to evaluate how accurate TRI’s program is at screening for depression.
The closer that TRI’s results are to the results of a MINI, the better the algorithm.
As we noted before, the current standard of care for screening for depression is through a questionnaire:
These questionnaires were developed in the 1990s and are still being used today as the current standard of care.
According to a study published in 2016 by US healthcare company Kaiser Permanente, this questionnaire missed around 50% of the depressive cases.
This means that only around 50% of the time that someone answers this questionnaire they will be correctly identified as having depression.
Ultimately we want to see TRI significantly improve upon the current standard of care as it will go a long way towards marketing approvals from the FDA.
How does today’s news affect our Investment Memo
This news today sees TRI complete our Objective #1 for the company.
TRI has published results that fit into our “Base Case” scenario for the company.
Ultimately, we think these results are good enough for TRI to go on to additional clinical validation studies in a larger cohort of patients, to ultimately satisfy the FDA conditions on commercialisation approval.
Objective #1: Complete Phase 2 clinical trial, release results
TRI is currently conducting a Phase 2 trial in the US on its algorithm for detecting current Major Depressive Episode (cMDE). Final results are expected in the June quarter (before July 1st 2024).
Milestones
✅ Complete recruitment of 400 patients
✅ Phase 2 Clinical Trial Results
🔲 Additional clinical validation studies
What do we want to see from TRI: TRI Investment Memo - May 2024
The news published today also mitigates the company’s clinical trial risk, as the interim results published in February were now confirmed.
Clinical Trial Risk
Clinical trial outcomes are never certain - TRI might not be able to generate results good enough for the regulator to approve the product. Clinical trials, even low cost ones, require capital in order to be conducted. A clinical validation study, which TRI may also have to complete could cost additional money.
What could go wrong? TRI Investment Memo - May 2024
What’s next for TRI?
Objective #4: Complete Pivotal Study
Today we are adding a new objective to the TRI Investment Memo. Now that it has completed its Phase 2 trial and published the results, the company will look to complete a Pivotal Study.
We had not yet added it as an objective as it was conditioned on the data collected by the company, however it appears that this Pivotal study will be the best way forward for the company to achieve FDA approval.
Milestones
🔲Complete Pivotal Study Design
🔲Appoint CRO for Pivotal Study
🔲IRB Approval for Pivotal Study
🔲Commence Pivotal Trial
🔲Interim Results
🔲Complete Recruitment
🔲Final Results
This study will set the stage for the FDA approval which is our #2 Objective for the company.
A reminder, why we Invested in TRI
Source: TRI Investment Memo 2 May 2024
- Screening for mental health issues from sleep data - novel solution to a serious problem. Current methods to screen for mental health are subjective and inaccurate, resulting in patients not receiving the right treatment. TRI is using AI to analyse sleep data to provide objective, accurate screening for mental health issues.
- Genuine AI (Artificial Intelligence) exposure - TRI didn’t just start working with AI once it became a popular investment thematic in recent months. TRI has a Head of AI, Dr Massimiliano Grassi, who has been doing AI for psychiatry for at least 6 years. TRI’s algorithm has advanced to Phase 2 trials and is getting better as time passes and more data is collected.
- ~$11M market cap - undervalued and unloved Medtech? - like our successful Investment in Oneview, our view is that TRI is undervalued at ~$11M considering the 9 years of investment into its tech & with a Phase 2 trial being run. We think this is a great entry after years of hard work with the right team to take TRI forward now in place.
- Imminent Phase 2 clinical trial results - 400 patients sleep data is being screened for depression using TRI. IF the trial result is positive, it could open the door to US FDA regulatory approvals and potentially even training the AI algorithm to screen for other types of mental health issues too. The final results are expected in the next couple of months. We think they will be a catalyst for TRI’s share price.
- Path to FDA approval - TRI has engaged with the FDA and the “De Novo” pathway was defined by the regulator, a type of FDA approval which is generally longer and more rigorous but reserved for genuinely new medical devices, which we think TRI’s algorithm is.
- USA based story and market - TRI has a US-based tech team and is going for the lucrative US healthcare market - which is the huge, clearly trodden pathway for big exits in biotech/medtech.
- Near term revenue opportunities (Stager) - US$15BN market - Commercial roll-out of TRI’s sleep staging software for sleep clinics - commercialisation is underway with licensing agreements and partnerships anticipated 2024. TRI’s novel products are aimed at sleep research organisations in the US, targeting the US$15 billion sleep medicine market.
- Proven new management team with key backers - recently appointed board and management team with significant success in US and global healthcare markets, including founding directors/financiers of Race Oncology ($600m+ market cap 2021) and ResApp Health, which Pfizer ended up taking over for ~$180M in 2022.
- Long term technical team retained (AI experts and human brain experts) - Neurosurgeon Dr Defillo and AI specialist Dr Grassi are the brains behind TRI’s tech - they’ve been involved together since 2019 and both bring a special skill set that we think will drive TRI’s success.
- Major shareholder is Fidelity Investments - one of the largest fund managers in the U.S, is the #1 shareholder in TRI with a ~10% holding in the company.
- Potential to screen for other psychiatric disorders - if it turns out that sleep data CAN successfully be analysed by its AI to screen for depression, it is possible that the AI can be trained to screen for OTHER mental health issues too.
- Potential to integrate TRI’s tech into wearables - TRI’s tech may potentially be integrated into wearables like a Fitbit, Apple Watch or Oura Ring that all track simplified sleep data. That’s a big commercial opportunity in our eyes.
Source: TRI Investment Memo 2 May 2024
Our TRI Investment Memo
You can read our Investment Memo in the link below.
This memo provides a short, high-level summary of our reasons for Investing.We use this memo to track the progress of all our Investments over time.
In our TRI Investment Memo, you can find the following:
- What does TRI do?
- The macro theme for TRI
- Our TRI Big Bet
- What we want to see TRI achieve
- Why we are Invested in TRI
- The key risks to our Investment Thesis
- Our Investment Plan

General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

