TRI: USA Veterans Affairs to start 12 week trial to screen for mental health issues. First trial with wearables…
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 18,820,000 TRI shares at the time of publishing this article. The Company has been engaged by TRI to share our commentary on the progress of our Investment in TRI over time.
Could your smart watch soon give you advance warning of potential mental health issues?
Our Investment, the $9M capped Trivarx (ASX:TRI), is aiming to dramatically alter the way we approach mental health - and it's through sleep.
TRI is aiming to bring the objective detection and screening of mental health conditions to the massive wearables market using AI.
TRI has an algorithm which it has already shown can accurately detect what is called a “current Major Depressive Episode (cMDE)” from a person's sleep data.
A current Major Depressive Episode is a technical term for a period of at least two weeks where an individual experiences a persistently low mood and/or a loss of interest or pleasure in activities, along with several other symptoms.
These periods have far reaching repercussions for patients, family members and friends.
TRI proved its AI algorithm is effective at screening for cMDE in July of last year when it successfully completed a Phase 2 trial.
To progress its wearables strategy, last week, TRI announced a collaboration with the US Department of Veterans Affairs (VA) for a clinical trial.
To fund this clinical trial, TRI just raised $2.25M (at 1.5c with a 1:2 attaching option).
The trial is going to give TRI valuable data that will help it unlock commercialisation opportunities.
TRI’s new trial will be led by Dr. Jennifer Martin, a highly respected expert in insomnia disorder and mental health.
Dr. Martin is one of America’s leading scientists on the nexus of sleep and mental health, the exact field that TRI works in.
What’s different in this trial?
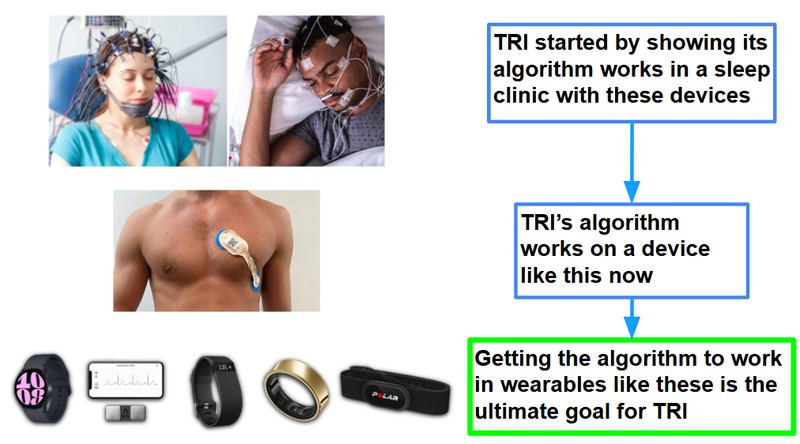
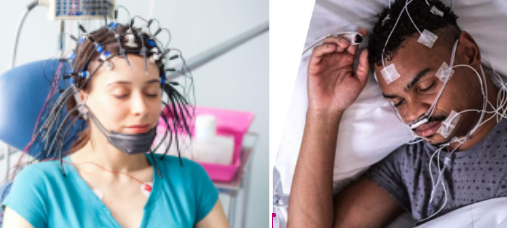
TRI’s previous trial data was generated from sleep clinics, where brain waves (EEG) and heart rate and heart rate variability data (ECG) were measured with devices that look like this:
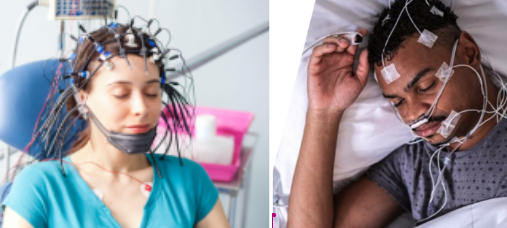
In this new clinical trial, TRI will look to prove that it can actually collect much simpler ECG data and have its algorithm work in the “real world".
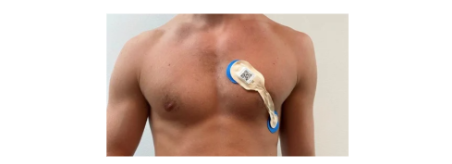
(No massive helmets in a sleep clinic, no multiple strips of pads connected to the chest)
In addition to using the single-lead ECG signal, patients will also wear a wrist-worn wearable device during the night - providing the company with additional data for further R&D and commercialisation.
The upshot for us as long term TRI Investors, is that we may now have a direct pathway to a major clinical validation of its technology through this trial.
We see this is a critical next step in proving its technology can be applied to mass market wearables like smartwatches.

(even easier to collect data - and used by hundreds of millions of people)
Once TRI’s new trial starts, we should have results in just 12 weeks
We like this trial because it's quick, fairly cheap, and it allows TRI to collect real world data from wearables - a large and growing market worth US$84.2BN last year (Source).
The ultimate vision for TRI is to get its mental health screening technology into smartwatches and wearables like Apple Watches, Oura Rings and Fitbits.
Last year there were 500 million wearable devices sold (Source).
And TRI is going to soon be working on its wearables R&D, with clinical trial data generated by one of the most renowned experts on the interaction between sleep and mental health.
TRI’s tech may also be able to eventually expand into disorders like depression, anxiety, bipolar and PTSD.
These are unfortunately very common disorders (and are also massive markets for treatment) so we hope to one day see TRI’s tech applied to them.
TRI now funded for the upcoming trial
Off the back of last week’s news, TRI went straight into a trading halt and raised $2.25M at 1.5c.
(the offer came with 1:2 free attaching options).
Post this capital raise, TRI will have a market cap of ~$9.2M.
The cash will be used to fund the VA trial and ongoing R&D activities (we think likely focusing on wearables).
We think it was a good idea for TRI to announce the Veterans Affairs trial and raise $2.25M amount at the same time, because there is now a short-term catalyst to look forward to without the threat of a potential capital raise hanging over the stock.
Capital raise overhangs can limit how much the market reacts to catalysts from a company, so getting it done and out of the way before big news is delivered is usually good for any company.
The trial is designed to test whether TRI’s sleep algorithm works with a “single lead ECG” which some wearable devices are capable of measuring.
As mentioned above, a key point we noticed from Thursday’s announcement - “patients will also wear a wrist-worn wearable” during the trial...
This is the first time TRI will be getting data concurrently from single lead ECG and a wearable during a clinical trial.

(Source)
How TRI’s tech could change the way we approach mental health
Right now TRI’s algorithm can detect major depressive episodes when using specific sleep monitoring devices...
But the real blue sky is if TRI’s algorithm works in wearables - the mass market.
TRI’s algorithm has already shown remarkable accuracy at detecting a current Major Depressive Episode (cMDE).
In July last year TRI put out results from a Phase 2 trial which proved its tech is effective at screening CMDE.
This trial data was generated from sleep clinics, where brain waves (EEG) and heart rate and heart rate variability data (ECG) were measured with devices that look like this:
The results showed that compared to a Phase 1 trial, “Sensitivity” increased from 71.6% to 87%.
Sensitivity is the measure of the ability of the algorithm to identify patients with the disease.
The current standard of care has a less than 50% ability to correctly identify a patient with the condition. (Source)
(For context, a survey is the current standard of care to screen for depression)
The current way of doing things is hardly “revolutionary”, given decades of advances in technology and health care.
But TRI has a potentially groundbreaking way to identify mental health issues - that could eliminate the need to do an unreliable manual survey on how you think you are feeling.
TRI highlighted the deficiencies of the current standard of care in a November 2023 presentation:

(Source)
So potentially less than 50% of the time the current standard of care gets it wrong...
Meanwhile TRI’s algorithm has an 87% chance to identify a patient with the condition.
Then more recently in November of last year, TRI was able to show that its tech was able to work off a “single lead ECG”.
This is what a single lead ECG looks like:
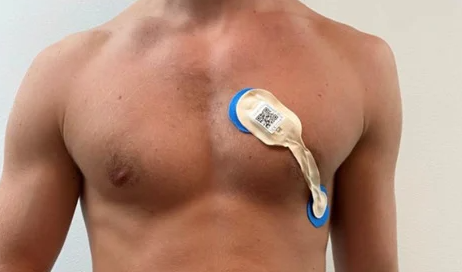
Many smartwatches/wearables are capable of collecting ECG data.
Some of the most familiar wearables look like this:

Since we Invested in TRI in May of last year we’ve seen the company take a number of positive steps towards being able to screen for depression using wearables.
This is a big reason for our Investment and where we think this clinical trial can help.
Why we like this upcoming clinical trial
Here’s why we like this upcoming Veterans Affairs trial:
- TRI gathers important data about whether its algorithm works with a single led ECG in the “real world”.
- This data can be used to help find a commercial to partner to fund a larger clinical trial
- TRI builds a strong relationship with a top institution and well respected leader in the fields of sleep and mental health.
And TRI now has the funds to complete the trial, following last week’s capital raise.
Again, here is the high level summary of what TRI had accomplished so far, and what this trial is seeking to do in the future:

More on TRI’s algorithm and what we are looking for from the VA trial
TRI’s algorithm detects major depressive episodes by measuring the difference between heart rate (HR) and heart rate variability (HRV) while a person is sleeping.
TRI has worked to show that patterns in data for these two measurements to be a “biomarker” (a biological signal) of mental health issues.
As we mentioned before, last year TRI published a 400-patient Phase 2 clinical trial that showed how its technology is applicable in designated sleep clinics.
Last year TRI adjusted its algorithm so that it would work by collecting and measuring the difference between a person’s heart rate and their heart rate variability.
This was a big breakthrough for the company because it unlocked the potential to apply its technology just to wearable devices.
(generally wearables don’t track brainwaves, but they do track a person’s heart rate).
This will be the first clinical trial that TRI will do to measure this using both a single lead ECG AND with wearable technology.
Since we Invested, we have been saying that screening via smartwatches and wearables is our long term “blue sky” upside for TRI.
And by conducting this trial with US military veterans, TRI believes it could unlock significant commercialisation opportunities.
TRI will conduct a 60-patient clinical trial with the VA over a 12 week period.
What we are looking for is:
- Can TRI replicate the same sensitivity and specificity that was previously collected?
- Is TRI able to validly collect ECG data from a single-lead ECG signal?
- Is TRI able to validly collect ECG data from wearable devices?
If it is able to do this, we see this clinical trial as a successful and valuable exercise.
Expert in insomnia disorder and mental health to lead the trial
TRI’s new trial will be led by Dr. Jennifer Martin, a highly respected expert in insomnia disorder and mental health:

(Source)
In terms of sleep and mental health - this is THE US guru of the science.
Dr. Martin was a recent past president of the American Academy of Sleep Medicine (AASM) and the AASM Foundation.
Dr Martin is also board-certified in behavioural sleep medicine by the American Board of Sleep Medicine (ABSM)
She also holds multiple prominent other roles, including Associate Director for Clinical and Health Services Research at the VA Greater Los Angeles Healthcare System's Geriatric Research, Education, and Clinical Center.
She is also a professor of medicine at the David Geffen School of Medicine at UCLA.
Effectively, Dr. Martin is one of America’s leading scientists on the nexus of sleep and mental health, the exact field that TRI works in.
So not only will this trial provide TRI another chance to further improve on past results, but it may also provide the important validation (from a prestigious scientist) that commercialisation partners might be interested in.
A leading principal investigator helps - and being connected to the US military is often seen as an important marker of a product's quality.
Good enough for vets - good enough for the rest of us perhaps?
And will a major commercial partner step out of the woodwork to help TRI join the dots on sleep and mental health?
(We hope so)
Here’s what we are looking for next from TRI...
What’s next for TRI?
🔄 NEW: US Veterans Clinical Trial
TRI’s new trial aims to recruit 60 patients across multiple VA sites, with study completion expected within 12 weeks from commencement.
First TRI will need to formalise the arrangement with the VA, and get the study parameters approved by the VA’s Institutional Review Board.
From there we can expect results within a 12 week period.
Objective #1: Complete Phase 2 clinical trial, release results
TRI is currently conducting a Phase 2 trial in the US on its algorithm for detecting current Major Depressive Episode (cMDE). Final results are expected in the June quarter (before July 1st 2024).
Milestones
✅ Phase 2 clinical trial results
🔄 Potential additional clinical validation studies
Source: “What do we expect TRI to deliver? -TRI Investment Memo (2 May 2024)
🔲 Complete a pivotal study
TRI has indicated after it published Phase 2 trial results, the company will look to complete a Pivotal Study.
TRI has already completed a Phase II trial with positive results and is ultimately seeking FDA approval for its depression screening algorithm via a Pivotal Study.
A “pivotal study” is usually a Phase 3 study, and TRI has previously indicated it intends to recruit 563 patients at at least 5 study sites to get the final round of data it needs to make a submission to the US FDA.
Successfully attaining US FDA approval or clearance would be the final hurdle for TRI to bring its product to market, and start making money from it.
This pivotal study will be the best way forward for the company to achieve FDA approval/clearance through the De Novo pathway.
After last week's announcement on the VA study, we ideally want to see a commercial partner or other organisation look to co-fund or fully fund this pivotal study.
Objective #4: Complete Pivotal Study
Milestones
🔲 Complete Pivotal Study Design
🔲 Appoint CRO for Pivotal Study
🔲 IRB Approval for Pivotal Study
🔲 Commence Pivotal Trial
🔲 Interim Results
🔲 Complete Recruitment
🔲 Final Results
Source: “What do we expect TRI to deliver? -TRI Investment Memo (2 May 2024)
What could go wrong?
TRI is a micro cap company that is not generating any revenue.
To advance its technology, TRI will need to complete more trials.
Like everything, this will cost money - this can either come from commercial partnerships or from capital markets.
To fund the upcoming trial and working capital TRI just completed a capital raise for $2.25M.
Given TRI’s previous trials have been low cost, we’re of the view that the VA trial will also be low cost.
TRI has previously indicated that it is looking to “advance potential sponsored clinical trials” as well as “testing and licensing opportunities”. If these opportunities take longer to negotiate, then TRI may need to raise capital in the medium term.
Funding/Dilution Risk
Small caps often need to raise cash to fund their growth. TRI is not generating any revenue and may need to raise capital, potentially at a discount. Capital raises may dilute existing holders.
Source: “What could go wrong” - TRI Investment Memo 2 May 2024
“Clinical trial risk” is also a key risk in the short term.
TRI may not be able to achieve the results it is seeking in the upcoming clinical trial which could cause a re-rate lower in TRI’s share price:
Clinical trial risk
Clinical trial outcomes are never certain - TRI might not be able to generate results good enough for the regulator to approve the product.
Clinical trials, even low cost ones, require capital in order to be conducted. A clinical validation study, which TRI may also have to complete could cost additional money.
Source: “What could go wrong” - TRI Investment Memo 2 May 2024
Our TRI Investment Memo
You can read our Investment Memo in the link below.
This memo provides a short, high-level summary of our reasons for Investing.We use this memo to track the progress of all our Investments over time.
In our TRI Investment Memo, you can find the following:
- What does TRI do?
- The macro theme for TRI
- Our TRI Big Bet
- What we want to see TRI achieve
- Why we are Invested in TRI
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

