TRI announces smartwatch and wearables screening for mental health issues.
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 18,820,000 TRI shares at the time of publishing this article. The Company has been engaged by TRI to share our commentary on the progress of our Investment in TRI over time.
They’ve done it.
TrivarX (ASX:TRI) has just announced success in the first step for screening for mental health disorders using ‘wearable’ technology.
We are talking about devices like Fitbits, Apple Watches or Oura rings.
Imagine being able to screen for potential mental health issues at home while you sleep wearing your smartwatch.
TRI is working to make it possible.
Today’s advancement is a big step forward in this space - and it has broadened patient reach and market opportunities significantly.
An ECG is an electrocardiogram - a test that records the electrical signals in the heart.
“Single lead ECG” is how smartwatches and wearables collect data.

There were at least 500 million wearable devices sold this year alone. (Source)
We have been saying that screening via smartwatches and wearables is our long term “blue sky” for TRI.
It opens up a significantly larger market.
And we weren’t expecting progress on the wearables front so soon from TRI.
Here’s a quick summary of the lead up to today’s TRI news and why this new development is so important:
TRI’s artificial intelligence technology can currently screen for mental health issues by analysing a patient’s sleep data.
Rich and detailed (brainwave) sleep data is collected in sleep clinics - this requires a bunch of electrodes stuck to the patients head, plus a heart rate monitor:
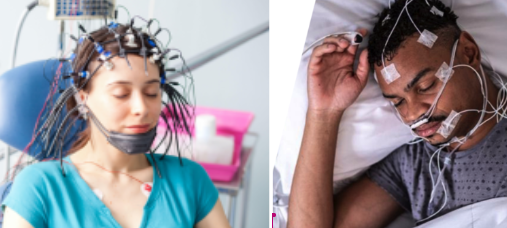
TRI has already had success in a Phase 2 clinical trial for depressive episodes from the rich sleep data (read more here)
This means that TRI has already identified the specific brain signals for depressive episodes when studied in conjunction with heart data.
TRI then tested if they could accurately identify depressive episodes WITHOUT the brain signals that are captured in a sleep clinic.
(from the simplified ECG data that can be collected by smartwatches and wearables.)
Turns out yes, it can - today TRI has reported promising results on initial testing, delivering sensitivity of 87% and specificity of 67% (more on what specificity and sensitivity later in this note).

As you can see, this looks much easier than having all those electrodes taped to the head.
Screening using wearables broadens the market significantly because people DO NOT need to go to a sleep clinic and strap electrodes to their head.
And many people are already currently using wearables anyway, so no change in behaviour is required.
TRI can now move out of sleep centres and into consumer wearables, sport and performance, military, cardiology monitoring.
Today, TRI said they are now working to advance sponsored clinical trials as well as testing and licensing opportunities.
We think wearables manufacturers would likely be interested in expanding their products’ functionality.
So TRI is now closer to proving the pathway for how to use smartwatches and wearables to screen for major depressive episodes.
When we first Invested in TRI - we highlighted this potential major upside:
(Source May 2024)
What about other mental health conditions beyond depression?
So can they do it for other mental health issues like anxiety, PTSD, bipolar, Alzheimer's, etc?
(all huge markets)
Quite possibly, we think it could look like this:
- Use Artificial Intelligence on detailed sleep data (biometric data, EEG and ECG recordings) from sleep clinics (EEG requires all those head electrodes) to identify the specific brain signals correlated to a particular mental health disorder.
- Prove it's accurate with clinical trials.
- Use AI to identify correlation to the same signal in the less detailed, but widely used ECG wearables data.
- Add the newly screenable mental health condition to the growing portfolio of mental health screenings.
Repeat steps 1 to 4 for as many mental health conditions as possible.
Then, ideally be acquired by one of the wearable makers - think Apple (Apple Watch), Google (Fitbit) or an up and coming smartwatch/wearable company.
TRI is still at the early stages and there is a lot of work to do, but today’s news is a very important milestone in being able to prove to potential partners and the market that success is possible.
Creating an AI technology that can be used in wearable technology was one of the big reasons for our investment in TRI.
And today the company took a big leap towards entering this US$61B market. (Source)
TRI’s new algorithm scored a sensitivity of 87% and specificity of 67% in detecting a current major depressive episode (cMDE).
These scores represent how accurate TRI’s algorithm is at successfully detecting someone with cMDE.
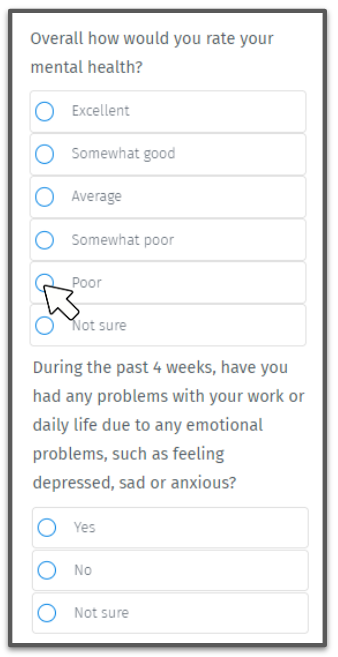
The current standard screening method has some drawbacks - and doesn't use any technology.
Often administered by nurses, a PHQ-9 is a manual questionnaire that has an accuracy rate of less than 50%, and there are lots of false positives (source).
So, for TRI to develop an algorithm with such strong initial results from existing data (collected earlier this year in the Phase 2 trial) is definitely a big positive.
Particularly because THIS algorithm only reads the biomarkers in the heart rate and heart rate variability, and NOT the biomarkers from brain waves.
This opens up the possibility for TRI to develop its algorithm specifically for consumer wearables or any other device that measures the kind of heart rate data used in the algorithm.
Wearable devices and AI algorithms: The next frontier for med-tech innovation
Sleep centres were the original market that TRI was targeting to establish clinical data, but the “blue sky” vision for the company was to develop a product for the much larger consumer wearables market.
Until today, TRI’s technology was limited by the fact that both brain activity AND heart rate / heart rate variability were needed for the AI algorithm to work.
Now that limitation is lifted and TRI can target the wearables market.
The wearables market is big - over half a billion wearable devices were sold last year, and the market is set to grow significantly:
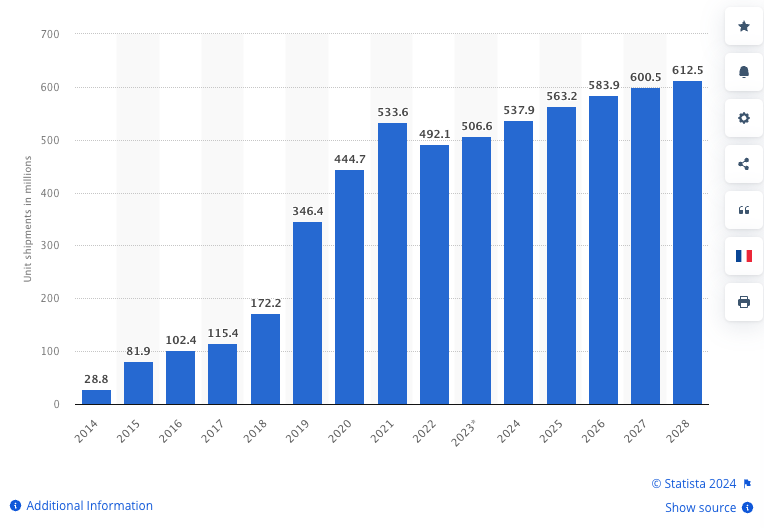
(Source)
The FDA has also recognised wearable devices as “single-lead electrocardiogram” (or SL-ECG) that can collect heart data for use in clinical trials.
A single-lead ECG uses only one electrode to record the heart's electrical activity from a single lead.
This type of ECG is typically used to monitor a patient's heart rate and rhythm over a short period of time.
This is what an Apple Watch uses:

(Source)
We think that if TRI can develop a mental health screening product specifically for wearable devices it unlocks huge potential in the consumer market.
Why did we invest in TRI?
We published the following reasons in our initiation note back in May 2024.
Major updates since then are noted in italics below.
1. Screening for mental health issues from sleep data
Current methods to screen for mental health are subjective and inaccurate, resulting in patients not receiving the right treatment. TRI is using AI to analyse sleep data to provide objective, accurate screening for mental health issues.
2. Genuine AI (Artificial Intelligence) exposure
TRI didn’t just start working with AI once it became a popular investment thematic in recent months. TRI has a Head of AI, Dr Massimiliano Grassi, who has been doing AI for psychiatry for at least 6 years. TRI’s algorithm has advanced to Phase 2 trials and is getting better as time passes and more data is collected.
3. ~$11M market cap - undervalued and unloved Medtech?
Like our successful Investment in Oneview, our view is that TRI is undervalued at ~$11M considering the 9 years of investment into its tech & with a Phase 2 trial being run. We think this is a great entry after years of hard work with the right team to take TRI forward now in place.
TRI’s market cap is now less - under $7M at last close.
4. Imminent Phase 2 clinical trial results
400 patients sleep data is being screened for depression using TRI. IF the trial result is positive, it could open the door to US FDA regulatory approvals and potentially even training the AI algorithm to screen for other types of mental health issues too. The final results are expected in the next couple of months. We think they will be a catalyst for TRI’s share price.
These are now published (read our take of the results)
5. Path to FDA approval
TRI has engaged with the FDA and the De Novo pathway was defined by the regulator, a type of FDA approval which is generally longer and more rigorous but reserved for genuinely new medical devices, which we think TRI’s algorithm is.
6. USA based story and market
TRI has a US-based tech team and is going for the lucrative US healthcare market - which is the huge, clearly trodden pathway for big exits in biotech/medtech.
7. Near term revenue opportunities (Stager) - US$15BN market
Commercial roll-out of TRI’s sleep staging software for sleep clinics - commercialisation is underway with licensing agreements and partnerships anticipated 2024. TRI’s novel products are aimed at sleep research organisations in the US, targeting the US$15 billion sleep medicine market.
8. Proven new management team with key backers
Recently appointed board and management team with significant success in US and global healthcare markets, including founding directors/financiers of Race Oncology ($600m+ market cap 2021) and ResApp Health, which Pfizer ended up taking over for ~$180M in 2022.
Former Resapp CEO Dr Tony Keating who spent significant time integrating Resapp into Pfizer, recently joined TRI.
9. Long term technical team retained (AI experts and human brain experts)
Neurosurgeon Dr Defillo and AI specialist Dr Grassi are the brains behind TRI’s tech - they’ve been involved together since 2019 and both bring a special skill set that we think will drive TRI’s success.
10. Major shareholder is Fidelity Investments
One of the largest fund managers in the U.S, is the #1 shareholder in TRI with a ~10% holding in the company.
11. Potential to screen for other psychiatric disorders
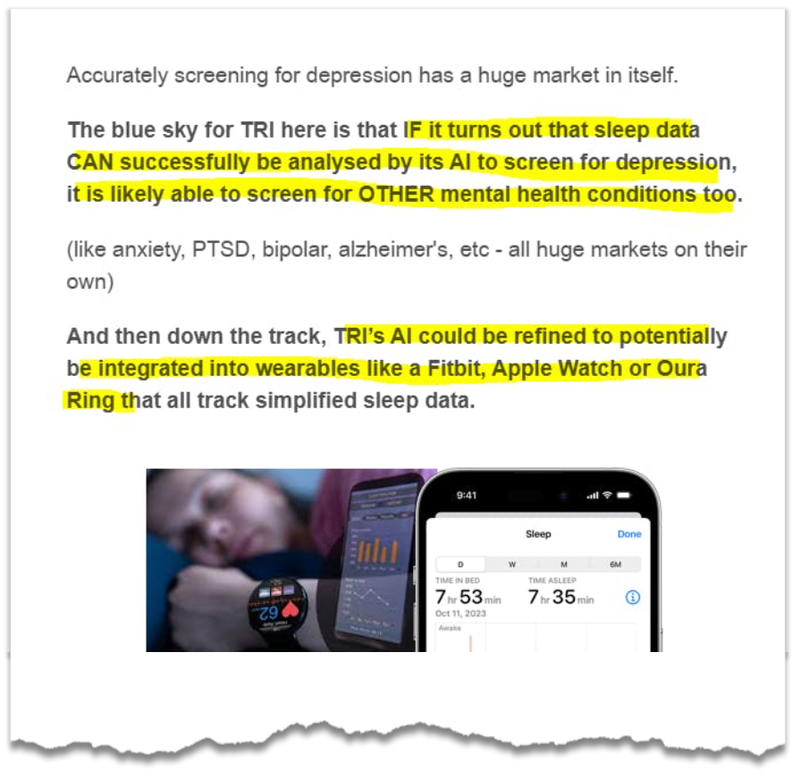
If it turns out that sleep data CAN successfully be analysed by its AI to screen for depression, it is possible that the AI can be trained to screen for OTHER mental health issues too.
12. Potential to integrate TRI’s tech into wearables
TRI’s tech may potentially be integrated into wearables like a Fitbit, Apple Watch or Oura Ring that all track simplified sleep data. That’s a big commercial opportunity in our eyes.
This is what today’s news was all about, we have seen some material progress here now.
The ResApp Story, what success looks like for TRI
ResApp developed a diagnostic tool that could detect and identify various respiratory conditions based on the sound of a cough made into a smartphone microphone using an algorithm.
TRI recently added Dr. Tony Keating to the board.
Dr. Keating brings a wealth of experience in securing approval from the FDA for algorithm based products.
Most importantly, as a co-founder of Resapp, Dr Keating has ‘been there and done that’ with an ASX listed biotech and been part of a team that delivered a successful exit to Pfzier.
ResApp began life as a University of Queensland based start up - and did a backdoor listing in 2015 when the share price was sitting at a market cap of ~$11M and had a share price of ~2c.
7 years later in 2022 the Pfizer acquired ResApp for ~$180M at a share price of 20.8c:
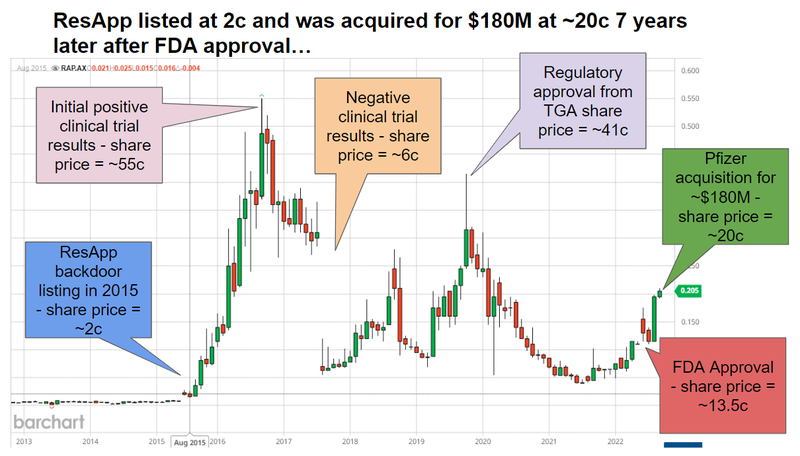
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
One of the key features of the ResApp was that it could be used on peoples phones.
As a consumer app, it appealed significantly to Pfizer that was looking to get in the market at the time (particularly around COVID-19).
We think that the wearable market is similar.
These devices, and health monitoring in general, will become more and more commonplace.
So for big pharma companies looking to enter the space, we think TRI presents as an attractive license partner.
What could a potential licensing deal look like for TRI?
In the announcement today TRI announced that it is looking to undertake a “sponsored” clinical trial and is evaluating “licensing opportunities”.
A licensing partner could come in the form of a big pharma company looking to advance their position in the wearable technology market.
OR, a wearable device manufacturer looking to advance their pipeline of software technologies and applications that can partner with the wearable device.
Either option would be a big win for TRI.
It can be difficult to value early stage biotech and med-tech companies just on the potential of the technology - particularly when the technology is incredibly novel.
TRI fits into this category - developing an AI algorithm to detect mental health conditions via biomarkers present in sleep.
To gauge the potential of this kind of product “external validation” like a licence agreement can be extremely useful.
Right now TRI is a technology play.
But we don’t know the scope of how big this technology could be for the market.
According to the World Health Organisation, around 1 billion people globally (that’s about 1 in every 8 humans) are living with some sort of mental health disorder, with anxiety and depression the most common.
But as we mentioned above, when it comes to measuring patient health, clinicians rely on the subjective PHQ-9 study.
This is very different to almost all other diagnostics tools out there.
We have glucose monitors for diabetes, heart monitors for cardiovascular disease.
... and a 10 stage questionnaire for mental health.
There is a big unmet need to improve in this area and we think that TRI has a viable solution.
Our TRI Big Bet:
“TRI re-rates to a $250M plus market cap on successful clinical trial results for screening one or more mental health disorders, FDA approval and/or is acquired for multiples of our Initial Entry Price”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our TRI Investment memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true. NOTE: FDA approval added to big bet 10 July 2024.
TRI’s results in more detail
Today, TRI announced that a newly developed algorithm can detect current major depressive episodes with 87% sensitivity and 67% specificity.
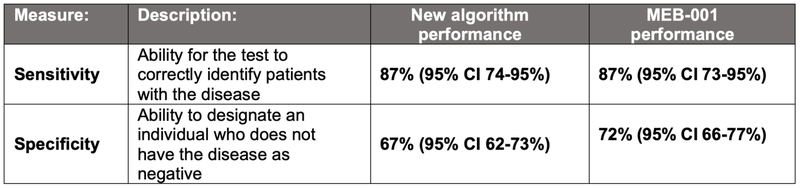
(Source)
The difference between this algorithm and the one that TRI used in a previous clinical trial is this algorithm can detect a current major depressive episode (cMDE) with a “single-lead electrocardiogram” (or SL-ECG).
(Note: no brainwaves (EEG) needed with this algorithm)
A single-lead ECG only uses one electrode to record the heart's electrical activity from a single lead and does not require brain data to work.
This means that TRI could develop this product for wearable technologies like smart watches that collect SL-ECG data from users.
The current standard screening method is a PHQ-9 questionnaire.
PHQ-9 has been around since the 90s and to date it remains the go-to screening method for depression.
The estimated accuracy of the questionnaire is not ideal, there are lots of false positives.
The sensitivity and specificity of the questionnaire is 85% and 85% respectively according to a meta-analysis conducted in 2021 - note: for cut-off scores greater than 10 (Source).
So for TRI to publish sensitivity of 87% and specificity of 67% we see as strong results, particularly considering that the algorithm couldn’t rely on the brainwaves variable from previous studies.
We won’t get into a debate about “optimal” cut-off scores - which are aggregations of patient answers on the PHQ-9.
But we do think if TRI’s algorithm can improve over time (especially on specificity), then its commercial appeal will improve further.
Importantly, the number of patients in TRI's study on the new algorithm was notably higher than at least half of the studies covered in the meta-analysis published in this well-cited article (which had a sample size of 55 or less):

(Source)
Our takeaway from the above article is that TRI’s new algo sits in the middle band of the sensitivities and specificities covered in the meta-analysis, but has the advantage of a much larger sample size than many of the studies.
This firms up TRI’s confidence in the algorithm, and hopefully, the confidence of regulators.
We’re now looking forward to what TRI can accomplish in the company’s next trial, as well as further improvement in the algorithm.
We think that this will be the next stage for TRI.
What’s next for TRI?
Objective #4: Complete Pivotal Study
Today we are adding a new objective to the TRI Investment Memo. Now that it has completed its Phase 2 trial and published the results, the company will look to complete a Pivotal Study.
We had not yet added it as an objective as it was conditioned on the data collected by the company, however it appears that this Pivotal study will be the best way forward for the company to achieve FDA approval.
Milestones
🔲 Complete Pivotal Study Design
🔲 Appoint CRO for Pivotal Study
🔲 IRB Approval for Pivotal Study
🔲 Commence Pivotal Trial
🔲 Interim Results
🔲 Complete Recruitment
🔲 Final Results
This study will set the stage for the FDA approval which is our #2 Objective for the company.
Other things that we are looking for include securing a licensing partner for the technology as well as further discussions with the FDA.
What could go wrong?
TRI is a micro cap company that is not generating any revenue.
In order to advance its technology TRI will need to complete more trials.
Like everything, this will cost money.
At the end of the September quarter TRI had $1.12M in the bank which is enough from a working capital perspective but perhaps not to run a full trial (we note that previous trials have been low cost, but we’re unsure how much the next trial will cost).
TRI has indicated that it is looking to “advance potential sponsored clinical trials” as well as “testing and licensing opportunities”, if these opportunities take longer to negotiate then TRI may need to raise capital.
Funding/Dilution Risk
Small caps often need to raise cash to fund their growth. TRI is not generating any revenue and may need to raise capital, potentially at a discount. Capital raises may dilute existing holders.
Source: “Risks” section - TRI Investment Memo 2 May 2024
Our TRI Investment Memo
You can read our Investment Memo in the link below.
This memo provides a short, high-level summary of our reasons for Investing.We use this memo to track the progress of all our Investments over time.
In our TRI Investment Memo, you can find the following:
- What does TRI do?
- The macro theme for TRI
- Our TRI Big Bet
- What we want to see TRI achieve
- Why we are Invested in TRI
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

