ILA clinical trial achieves anti-dengue activity in humans
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 3,766,666 ILA Shares at the time of publishing this article. The Company has been engaged by ILA to share our commentary on the progress of our Investment in ILA over time.
Phase 2a/b results are in...
Island Pharmaceuticals (ASX:ILA) has demonstrated its drug is active against the dengue fever virus in a human clinical trial.
“Results show that ISLA-101 demonstrated clinically meaningful anti-dengue activity, which included a material reduction in viral load and symptoms.”
ILA reported “meaningful reduction in both viremia (viral load) and symptoms in preventative cohort”
(meaning good for a pill that you take if you are in a high risk dengue location, so IN THE EVENT THAT you get dengue fever it reduces the symptoms - a huge market)
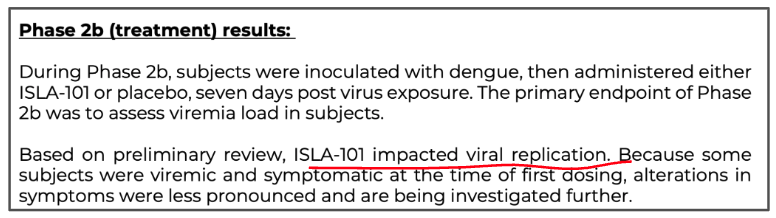
And that “Treatment cohort demonstrated signals of drug effect”
(meaning good early signs for a pill you would take if you have ALREADY CONTRACTED dengue fever, to reduce the symptoms - more info on this expected after ILA has had time to further analyse the results)
Anyone unfortunate enough to have had dengue fever will tell you it’s no joke - symptoms include sudden fever, chills, severe headache, and muscle/joint pain.
And in extreme cases... death.
Dengue fever infects around 400 million people each year.
It kills ~40,000 people each year.
Half the world’s population is at risk of getting it.
A quick google news search for “dengue” shows that in the last 24 hours there are more than usual outbreaks happening around the world - even in Australia.
(great timing for ILA to deliver their successful human trial results for their dengue fever drug)
There is currently NO specific prevention OR treatment currently for this virus... and cases are rising sharply.
ILA might be able to change that...
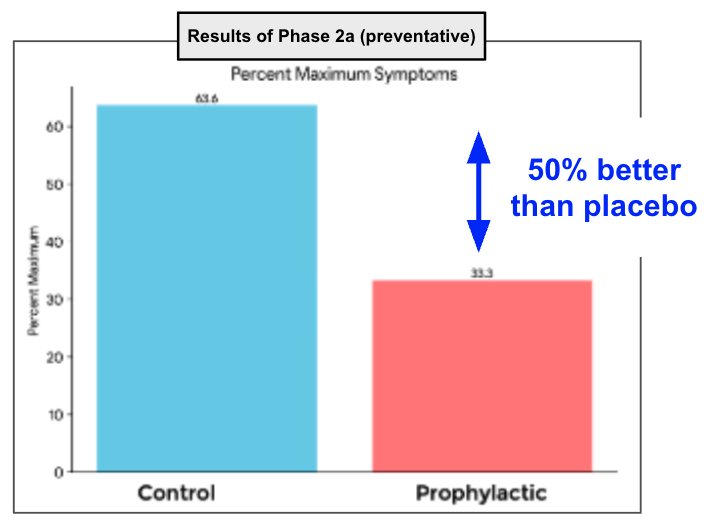
Now ILA has clinical data that shows that as a preventative:
- A reduction in viremia (viral load)
- Clinically meaningful reduction in symptoms
ILA also could have a treatment for dengue fever as well - ILA was able to show that its drug impacted viral replication.
ILA says it has already started more analysis of the data to establish if this meaningfully reduces symptoms:
ILA’s Managing Director Dr. David Foster will provide an update on the results via zoom on Tuesday 17th June at 11:00AM (AEST), you can register for the talk here:
REGISTER FOR ILA PHASE 2a/b TRIAL RESULTS CALL WITH DR DAVID FOSTER
There is also a pretty useful Q&A section to help understand the results starting on page 4 of ILA’s results announcement - read it here.
ILA’s strongest results are from the Phase 2a clinical trial, which tested whether ILA’s drug works as a preventative measure.
ILA will need to conduct more ‘advanced lab analyses’ to establish if there is a reduction in viral load and symptoms from the Phase 2b cohort (using its drug as a treatment for dengue).
While other companies have tried using small molecules to reduce the effects of dengue fever, ILA is the first company to actually demonstrate a benefit in a clinical trial.
These results set the stage for ILA to go down the preventative path, which means that the drug could be used by anyone visiting countries where there is a genuine risk of getting the disease.
(like malaria tables for travellers)
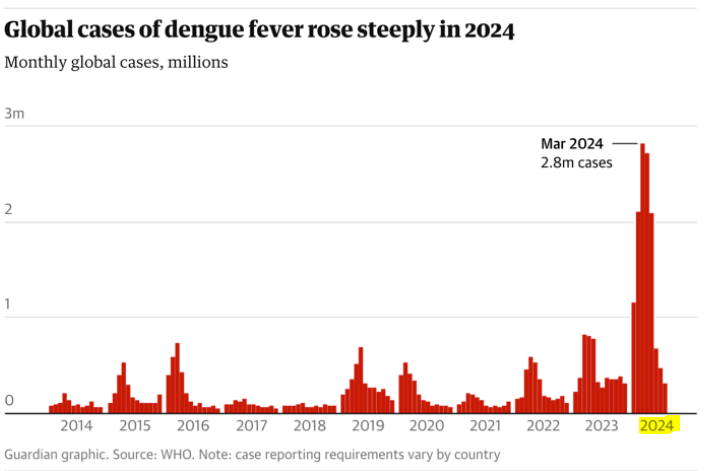
Cases of dengue fever continue to grow around the world, and things are only getting worse:
Even in Australia, dengue is becoming a big issue.
Last week the Australian government issued a travel warning for the Cook Islands as it declares a dengue fever outbreak.
And ABC recently reported on the first case of dengue fever in Cairns since 2018.

(Source - ABC news report first local case of Degue in Cairns since 2018)
Just over the last few days there have been countless articles warning travellers about dengue fever, particularly in popular holiday destinations.
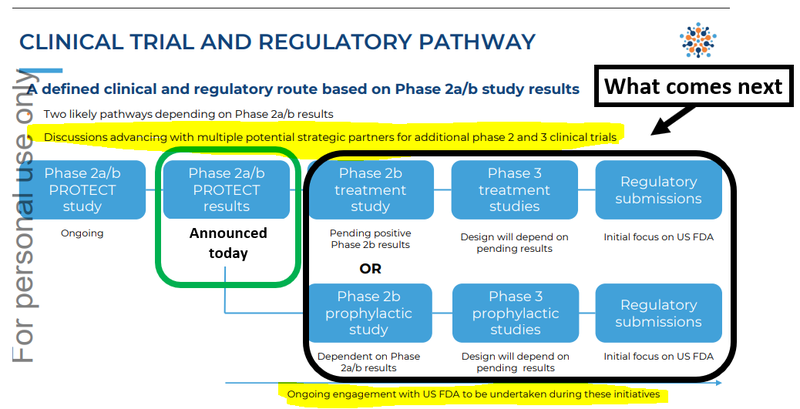
ILA will take the next few weeks to evaluate the clinical trial results and work with the FDA on what the next clinical trial will look like.
We expect ILA’s next clinical trial to evaluate whether its drug works with “wild dengue”.
This is dengue fever that a person catches ‘in the wild’ - the symptoms are much harsher and the virus more potent.
The Phase 3 clinical trial is the final hurdle before the drug can be sold into market:
If ILA succeeds in a Phase 3 clinical trial, it will be eligible for Priority Review Voucher (PRV) - which is like a bonus incentive for drug developers working on neglected tropical diseases.
This PRV is a transferrable asset that ILA can either use to speed up FDA approval of its next drug or onsell to another company looking for accelerated approvals.
PRV’s generally sell for between US$100M and $200M but some as high as US$350M.
The most recent sale only a weeks ago was for US$155M (A$241M).

(Source)
We did a deep dive on PRVs in our last ILA note here: Priority Review Voucher eligibility could be worth >US$100M
Strong trial data opens doors to partnerships?
One of the main reasons we think today’s news is so strong is because ILA now has clinical trial data it can shop around to potential partners.
ILA now knows:
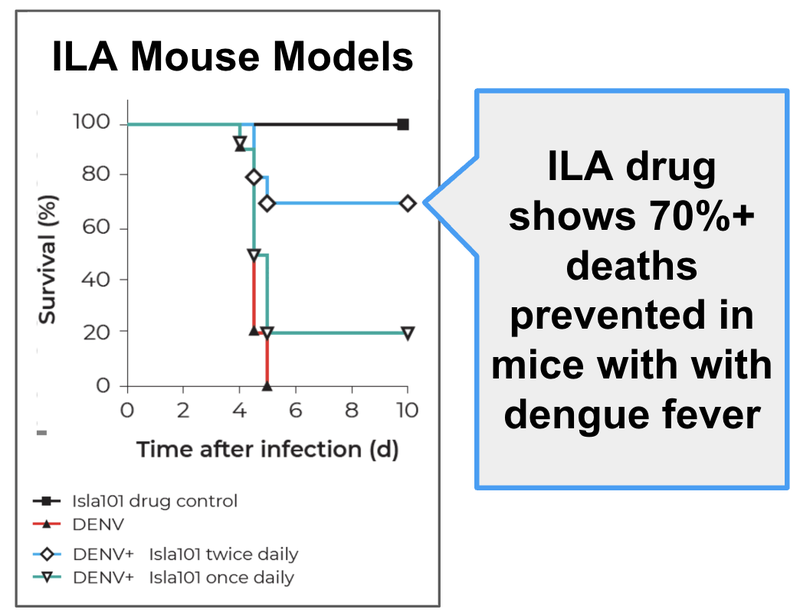
1. Animal models show the drug works against lethal doses of dengue - ILA has shown that mice infected with a wild strain of Dengue virus would all die within 5 days of infection. Whereas mice infected with the dengue virus BUT using ILA’s drug twice daily, would have a 70%+ survival rate.
2. ILA knows its drug is safe in humans - ILA’s drug is being repurposed for use against dengue virus. Before this ILA’s drug has had over 40+ Phase I, II and III human clinical trials completed and is recognised by the FDA as safe.
3. Now we know ILA’s drug works against a weakened dengue strain in HUMANS - today’s announcement showed that when using ILA’s drug as a preventative, it reduces symptoms of humans infected by a weakened strain of dengue by more than 50% relative to placebo. ILA’s drug also “materially reduced viral load” in patients...

We think these are all strong datapoints to have going into a Phase 2 or 3 trial...
Based on all of the above, partners might be willing to get exposure to ILA’s drug early by funding trial costs OR by signing licensing deals that are contingent on positive future trial data.
A few weeks back ILA’s CEO David Foster talked about these opportunities saying he was roadshowing ILA’s drug “in Singapore, Hong Kong, Australia and the US”...
As well as Brazil where ILA was “ taking part in a pharmaceutical partnering conference”.

Securing commercial partners for a drug that is still in the development stage is one of the holy grail announcements for any pre-approval biotech.
We think that the stage is set for the company to surprise the market with a partnering/licensing deal for a larger Phase 3 trial.
Particularly due to rapidly rising dengue cases in some countries...

(Source)
A partnership deal is one of the key objectives we listed as part of our ILA Investment Memo:
Objective #3: Partnership for Phase 2/3 trials
Pending the results of Objective #2, we want to see ILA lock in some sort of partnership deal to help fund its phase 2/3 trials.
Milestones
🔲 Partnership deal
Source: What do we expect ILA to deliver? - ILA Investment Memo 21 May 2025
Island Pharmaceuticals
ASX:ILA
9 key reasons why we are Invested in ILA
1. Big addressable market - dengue infects up to 400M per year, kills ~ 40k
Infectious diseases are usually undertreated. ILA’s current focus is dengue fever which infects ~100-400M people and kills ~36k people per year.
The dengue fever treatment market is worth ~US$2.14BN and expected to grow to US$3.66BN by 20234.
2. Dengue fever cases are growing globally, but no specific treatment or preventative
Globally, cases of dengue have doubled each year since 2021, and nearly tripled in 2024.
In April last year there was an emergency declaration by the CDC about rampant dengue fever in South East Asia and South America.
The US government and the US military have acknowledged that dengue fever is a major health crisis too.
Given there is no treatment or preventative, outbreaks could have disastrous consequences for populations.
3. ILA is backed by the Race Oncology team (went from 6.6c to $4 per share - a 60 bagger)
Founder of Race Dr William Garner holds 15.79% of ILA. Previous Chief Scientific Officer of Race, Dr Daniel Tillet holds 6.72% of ILA and one of the founding directors of Race, Chris Ntoumpelous, is a non-executive director of ILA and participated in the recent placement at 15c.
4. Dengue fever clinical trial catalyst due in the next few weeks
Phase 2a/b clinical trials have all been paid for and completed.
Results are due inside the next few weeks, a positive outcome here will de-risk the drug’s efficacy and provide an indication whether ILA’s drug works and if it is better as a preventative or as a treatment.
UPDATE: Today’s announcement delivered these results
4. ILA could be eligible for “Priority Review Voucher” (PRV) - worth ~ US$100M
If ILA can get its drug FDA approved it could be eligible for a “Priority Review Voucher”.
A PRV means ILA can get its drug to market quicker. A PRV is transferable and has sold for on average ~US$100M (A$155M) in recent years.
If awarded, this PRV alone could be worth many multiples of ILA’s current market cap of ~$43M (fully diluted), ILA can choose to sell this PRV to raise a substantial amount of non-dilutive capital.
5. The active ingredient in ILA’s drug has already been proven safe in multiple clinical trials
ILA’s main drug candidate ISLA-101 has an active ingredient called fenretinide. There have been multiple clinical trials using this active ingredient for cancer and respiratory conditions in humans - providing a bounty of safety data for ILA.
This means that ILA has been able to ‘skip’ Phase 1 clinical trials as the important ‘safety’ hurdle has been de-risked (source - fenretinide data).
6. The US Government and the US Army both supported ILA’s clinical trials
The US government recently provided US$625k in grant funding for ILA’s clinical trial and the US Army provided clinical support under a CRADA (Cooperative Research and Development Agreement).
7. Possibility to expand drug to other infectious disease indications
IF ILA’s drug works for dengue fever, it could be applied to treat/prevent other infectious diseases like Zika, Yellow Fever and West Nile fever.
8. ILA to enter Biodefense space.
ILA has an exclusive option to acquire a drug that can be repurposed to tackle Ebola, Marburg, Zika - the kind of viruses that can be weaponised in war and conflicts.
This gives ILA potential exposure to the government/military anti-viral stockpiling thematic.
This drug could also be eligible for PRV and has animal data and human phase 1 safety data.
The rest of our Investment Memo is later in today’s email.
Before that, here is a quick overview on what ILA is targeting, what a priority review voucher is and why we think it's important for the ILA story and what we want to see next from ILA.
We are hoping that a combination of the above reasons come together to help ILA Achieve our Big Bet which is as follows:
Our ILA Big Bet:
“ILA re-rates to a $500M+ market cap by developing its dengue fever or Galidsiver drug”.
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our ILA Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
What’s next for ILA?
More data on Phase 2b clinical trial
In the announcement today, ILA says that more work needs to be undertaken to establish whether or not there is a clear reduction in symptoms when using its drug as a preventative.
The company will “actively advance lab analyses and provide updates, based on relevant findings”.
We think it will take the company a number of weeks to go through the data to establish whether the drug was effective as a treatment.
Phase 2/3 clinical trial design and completion for dengue fever
Now that the phase 2a/2b results are unblinded, we want to see ILA start designing its next trial for dengue fever.
The next stage will be a meeting with the FDA (these generally take about 4-6 months) and then a clinical trial design for the next phase of trials.
Here are the milestones we will be tracking:
🔲 FDA meetings to determine endpoints for the trial
🔲 Clinical trial design completed
🔲 Clinical trial starts
🔲 Clinical trial completed
🔲 Clinical trial results

Galidesivir acquisition
We also want to see ILA make a decision on the option it has to acquire Galidseivir.
ILA is close to securing an exclusive option to acquire a drug called “Galidesivir” that is currently owned by US$2.1BN, NASDAQ listed BioCryst.
That drug has existing non-human data for Marburg disease and two Phase 1 safety studies.
Marburg is a severe disease with a fatality rate of up to 88% - it also has no specific treatment or vaccine at the moment.
The upside for ILA here is that the drug it would be acquiring could be eligible for the FDA’s “animal rule”.
The animal rule is when drugs are approved by the FDA using animal data alone because running human trials are considered to be unethical OR non feasible.
We think this could be just as interesting as ILA’s dengue fever drug...
Check out our deep dive on Galidseivir here: ILA could also become a biodefense “stockpiling” play
Here are the milestones we are tracking for Galidesivir:
🔲 Option exercise
🔲 Forward plan
What are the key risks?
ILA had positive data from its Phase 2a clinical trial, but the full set of data for the Phase 2b is yet to be analyzed.
It is not clear yet whether the Phase 2b has met the primary endpoint of “reduced viral load” and the company will need to analyse the data further to establish whether the primary endpoint was met.
There is still a risk that ILA is not able to show that the primary endpoint is met in the Phase 2b trial, this would reduce the company’s optionality when it comes to designing the Phase 2 / 3 clinical trial on wild dengue.
Clinical trial risk
It is important to be aware that clinical trials can be unsuccessful.
Here are some of the standard risks that are associated with biotechs that are undertaking clinical research:
Patient recruitment is delayed or fails
Ethics approval is delayed or fails
Clinical trial cost blowouts
The drug or treatment is ineffective at treating the particular disease (usually determined by clinical trial results in Phase II and Phase III)
The design of the trial is such that the regulatory body does not approve the drug/treatment.
There is a chance that one or more of ILA’s clinical trials fail to meet their primary or secondary endpoints, meaning the treatments fail to satisfy the criteria of the studies.
Any clinical trial results, if negative, could hurt the ILA share price.
Source: ILA Investment Memo: 21st May 2025
Our ILA Investment Memo:
You can read our ILA Investment Memo in the link below. We use this memo to track the progress of all our Investments over time.
Our ILA Investment Memo covers:
- What does ILA do?
- The macro theme for ILA
- Our ILA Big Bet
- What we want to see ILA achieve
- Why we are Invested in ILA
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

