Genetic Technologies shares rocket after cancer tests validated
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Shares in Genetic Technologies Ltd (ASX:GTG; NASDAQ:GENE) surged nearly 60% on Friday after the company announced two new ground-breaking cancer risk assessment tests had been completed and validated.
Genetic Technologies is a diversified molecular diagnostics company that has already had success with cancer risk detection products that it has launched.
The current tests just validated are branded as ‘GeneType for Colorectal Cancer’ and ‘GeneType for Breast Cancer’.
Next generation risk assessments combine multiple clinical and genetic risk factors to better stratify individuals at increased risk of developing disease.
With regard to the group’s colorectal cancer breakthrough, it incorporates the most impactful risk factors in order to define an individual’s risk of developing colorectal cancer, so the healthcare provider can make screening and preventative care recommendations that are tailored to their patient’s personalised risk.
Colorectal cancer rates increasing
Colorectal cancer is the third most commonly diagnosed cancer in the US, yet 1 in 3 adults are not receiving the appropriate colorectal cancer screening for their age.
In addition, rates of colorectal cancer among 20-49 year olds are steadily increasing.
Identifying patients who are most at risk for colorectal cancer can lead to enhanced screening protocols and better outcomes.
Most individuals diagnosed with colorectal cancer do not have a significant family history of the disease.
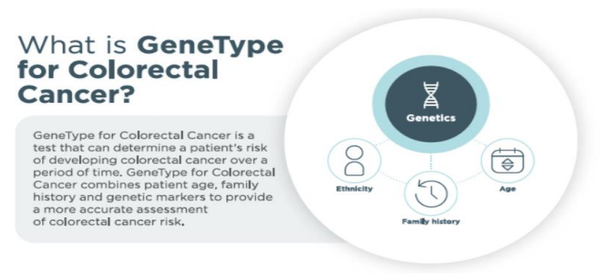
‘GeneType for Colorectal Cancer’ evaluates the genometric risk of developing colorectal cancer for men and women over age 30 who do not have a known pathogenic gene variant.
In sporadic colorectal cancer, no single gene mutation is causal of disease.
Rather, common DNA variations, called single nucleotide polymorphisms (SNPs), each contribute a small but measurable risk of developing disease.
‘GeneType for Colorectal Cancer’ analyses a patient’s DNA for more than 40 SNPs that have been clinically validated in their association with colorectal cancer.
By combining the effects of all of these SNPs into a single polygenic risk score (PRS), ‘GeneType for Colorectal Cancer’ provides a superior risk stratification over standard risk assessments that incorporate only clinical factors.
‘GeneType for Colorectal Cancer’ is clinically validated for men and women 30 years of age or older and for individuals of Caucasian descent.
Genetic Technologies sees the scope for continual improvements in its tests, and with the opportunity to add fully validated models for additional ethnicities the company’s growth profile continues to improve.
Further, the robust share price momentum displayed on Friday could well continue as the group’s technologies are demonstrated to have applications in larger markets.
New test increases coverage to 95% of women
Genetic Technologies’ latest progress in detecting breast cancer risk is equally impressive, particularly given that it will be building on an already strong position in this field.
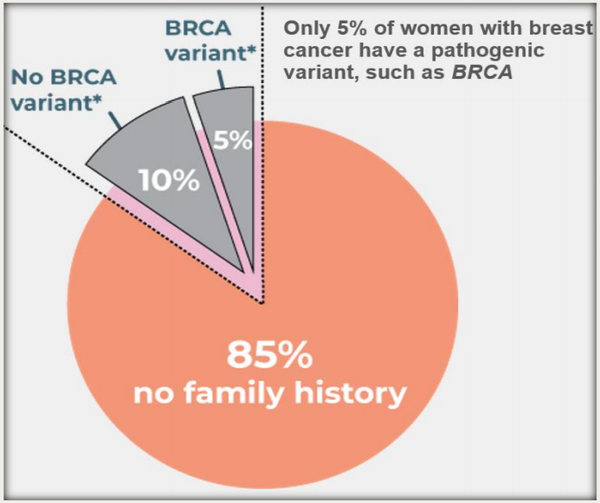
Germline genetic testing for mutations in BRCA1 and BRCA2 allows for the identification of individuals at significantly increased risk for breast and other cancers.
However, such mutations are relatively rare in the general population and account for less than 10% of all breast cancer cases.
The remaining 90% of non-familial or sporadic breast cancer have to be defined by other genetic/clinical markers common to the population at large and this is where Genetic Technologies has focused its attention.
The newly developed ‘GeneType for Breast Cancer’ test is aimed at risk detection of non-BRCA related sporadic breast cancer (that is, for those women who do not have an identified family history of breast cancer).
Importantly, this means that Genetic Technologies’ new test covers 95% of women.
Demand to be driven by affordability
Genetic Technologies’ new tests tick all of the boxes in terms of their scientific credibility, but the company has also maximised its opportunities of gaining market saturation by making the tests affordable to individuals, as well as positioning the tests to become part of regular health care provider health checks and a core component of government health cost reduction programs.
Economic benefits of early detection are compelling, particularly given the high costs of treatment from late stage diagnosis.
The added incentive for healthcare providers is that the tests provide a basis for the initiation of lifestyle changes that can reduce both types of cancer.
With a strong global patent portfolio, Genetic Technologies is poised to enter new markets, with one of the most recent prominent developments being the establishment of collaborative agreements with Chinese partners which should provide assistance in terms of technological development, as well as opening up a multi-billion dollar market.
Management intends to introduce the new ‘GeneType for Colorectal Cancer’ and ‘GeneType for Breast Cancer’ genetic tests to healthcare providers through a global network of distribution partners.
The company has already announced it is working with TGen in the United States and also Chinese partners connected to the Hainan Free-Trade Zone.
More announcements are anticipated in this regard in coming months as the company moves to commercialise its suite of world-leading genetic tests.
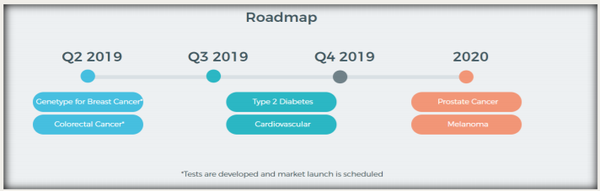
Following the successful completion of the ‘GeneType for Colorectal Cancer’ and ‘GeneType for Breast Cancer’ genetic tests, GTG’s highly credentialled scientific and product development division will refocus on the future pipeline of new tests as detailed below.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.




