CPH to expand US footprint after USOTC listing
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Creso Pharma Ltd (ASX: CPH) announced today that it has successfully completed a dual listing in the US and will commence trading in the region when the market opens US time (around 7.30pm this evening AEST).
The company is trading on the US OTCQB under the code COPHF.
The listing gives CPH greater exposure to a deeper capital market as well as greater access and liquidity for North American investors. Further to this, it provide additional opportunities to attract institutional and retail investors and for CPH to expand its investor base in the US.
The listing follows enormous interest from a large range of US investors as CPH has gradually built up a presence in the cannabis and psychedelics markets.
A US listing will allow CPH to fast track its move into psychedelics as the company looks to close out its acquisition of privately owned Halucenex Life Sciences.
Halucenex recently entered into a consultancy agreement with leading US-based scientific consultancy HeteroGeneity, LLC to progress a US market entry strategy for the potential use and licensing of its botanical psilocybin products and compounds.
Halucenex is focused on progressing clinical trials to research the efficacy of psilocybin to treat and alleviate Treatment Resistant Depression in individuals suffering from PTSD and other mental illnesses.
The company’s Phase II clinical trials will test the efficacy of psilocybin on the treatment of PTSD. Halucenex recently expanded the recruitment of its phase II clinical trial to include participants that have not served in the military or undertaken roles as first responders who are suffering from Treatment-Resistant Post Traumatic Stress Disorder (PTSD).
The PTSD market is valued at US$10.5 billion by 2025. This market could increase substantially if the user base extends beyond veterans.
One thing the OTC Dual Listing will provide CPH with in relation to psychedelics is greater exposure for clinical studies with universities, hospitals and research centres.
Halucenex has access to one of the largest supplies of synthetic psilocybin in Canada. Its short-term focus is to become a clinical drug pipeline provider.
The acquisition delivers CPH several multiple near-term catalysts:
- Entrance into a new, and potentially highly lucrative industry vertical, with additional potential future revenue streams in mental health.
- Licensing deals in North America and more broadly
- Clinical trials with a broadened scope in universities, hospitals and research centres
This move comes amid continued upturn in cannabis and psychedelics sectors.
A major legislative shift on cannabis in the US has drastically increased demand in the region, most notably with the Marijuana Opportunity Reinvestment and Expungement (MORE) Act being introduced to Congress, which would eliminate the federal ban on cannabis.
The dual listing provides easier comparisons to US and Canadian listed peers operating in the cannabis and psychedelic medicines sectors
Non-executive Chairman Adam Blumenthal commented, “The cannabis and psychedelic medicines sectors continue to gain considerable momentum.
Recent regulatory shifts have left the Company in a favourable position and Creso is well placed to become a global leader across both sectors.”
Fast tracking in the USA
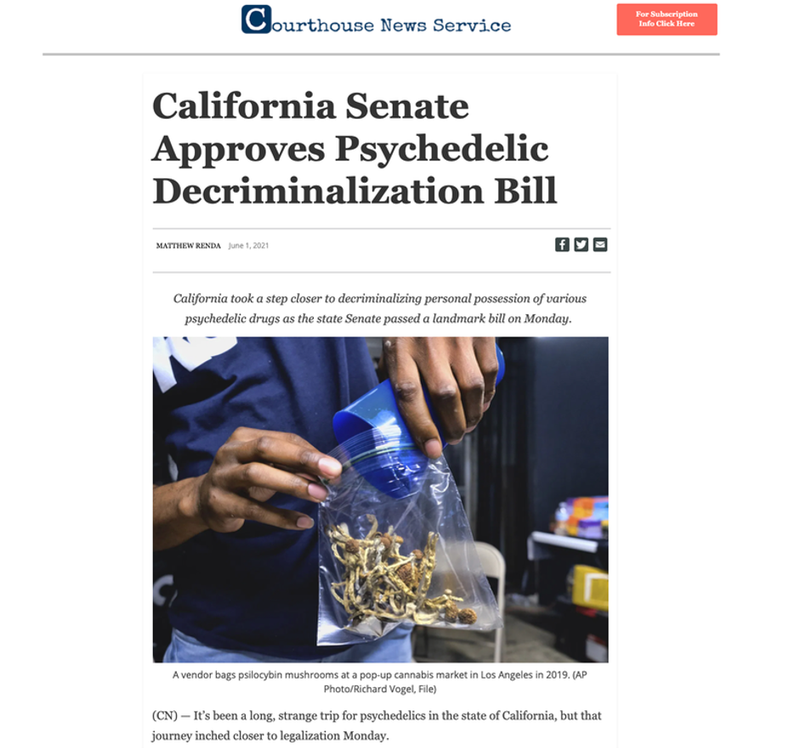
In what is a major development for CPH, Halucencex is fast tracking its go to market strategy in California.
This comes after the recent passing of Senate Bill 519 through the California State Senate, which if signed into law, would make a wide range of psychedelic substances including psilocybin legal to use and possess for adults over the age of 21.
The law is designed to combat growing mental health issues in the US and would mean amore health-focused approach to the use of psychedelic compounds to address this current mental health crisis.
To remove the stigma around psychedelics as a medical alternative, the law would also expunge prior criminal offences for use and possession.
If the bill passes as expected, it would open the doors for Halucenex to accelerate its development plan.
The market for PTSD therapeutics is expected to grow to US$10.5 billion by 2025, but this could increase substantially if the user base extended beyond veterans.
Mental illness in Canada more broadly has an economic burden of C$51 billion per annum, highlighting the large market opportunity for Halucenex and Creso Pharma.
CPH can also fast track its cannabis sales
While sales continue to grow through CPH’s wholly-owned subsidiary Mernova Medicinal Inc., it could see sales boosted further after it announced this week that North American partner, ImpACTIVE has finalised approval for CPH products to be stocked with America’s largest pharmacy chain, CVS Pharmacy.
CVS has outlets throughout the US. It is owned by CVS Health and is currently the largest pharmacy chain in the United States by number of locations. The group operates 9,900 retail locations in 49 states, as well as stores in the District of Columbia and Puerto Rico.
The deal could open up a market expected to be valued at US$22BN by 2022 and C$220M in Canada.
Read the full announcement here.
What to expect next from CPH
Here’s what we expect to happen next :
- Approval process for additional products underway – Creso to leverage development to progress agreements for its own range of CBD-based products
- Creso Pharma to assist ImpACTIVE with business development initiatives in the US to drive sales growth
- Both parties continue to collaborate on product development initiatives
- ImpACTIVE and Creso will target other large retail outlets via the RangeMe platform. Buyers from other large US retailers including Walgreens, Whole Foods, PetoCo, Target, GNC and Safeway amongst others.
- Creso Pharma is also working with ImpACTIVE to progress a number of new products, including topical rollers and creams containing only CBD, as well as THC and CBD combinations. The launch of these products is scheduled for the coming months with development currently in the final stages.
The OTC listing is a major milestone for CPH and we expect to see further sales growth in the cannabis space, as well as several catalysts over the next few weeks and months including the completion of the acquisition of Halucenex.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






