BOD: Clinical trials set to begin on chemist-available, cannabis insomnia treatment
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 3,535,112 BOD shares at the time of publishing this article. The Company has been engaged by BOD to share our commentary on the progress of our Investment in BOD over time.
Insomnia.
We’ve all probably had a few sleepless nights before.
With 10% to 30% of all adults struggling with chronic insomnia, the market to treat it is forecast to be worth US$6.3BN globally by 2030.
Existing options to improve sleep work to varying degrees (some dubiously).
But a new way to combat insomnia may soon be available at your local pharmacy, using cannabidiol (CBD) — the non-hallucinogenic component of cannabis.
A recent change by the Therapeutic Goods Administration (TGA) of Australia allows for approved, low-dose CBD products to now be sold over-the-counter (OTC) in pharmacies.
There have not been any CBD products approved yet for OTC sale in pharmacies - yet.
However our sub-$10M capped cannabis Investment BOD Science (ASX:BOD) is keen to be first to enter the national market.
BOD is at an advanced stage with its product, with a Phase IIb clinical trial to begin shortly, and a licensing agreement already in place so it can quickly start making sales.
The Phase IIb clinical trial is the final step in R&D for the product and it should provide sufficient data for application of a low dose CBD product with the TGA.
198 participants will be involved in the study over eight weeks, and the trial will be run by Australia's leading sleep research organisation, the Woolcock Institute.
Earlier in the month BOD reached a major milestone, completing the screening of trial participants, and randomisation will be completed by the end of the month.
If the trial is successful, it is almost certain to be first-to-market with a regulatory-approved CBD product.
In December BOD signed an exclusive licensing and five year supply agreement with Australia’s largest generic pharmaceutical and private label OTC medicines company, Arrotex Pharmaceuticals.
Under the agreement, BOD receives an initial $500,000 cash payment from Arroxtex and will receive revenue for supplying the product formulation and delivery method over the five year term.
Arrotex is Australia's largest supplier of generic drugs to pharmacists, with a network of 5,700 pharmacies nationwide. Here’s some of the likely recognisable brands it owns:

That means if clinical trials prove BOD’s insomnia treatment to be safe and effective, it is more than likely you will soon see a BOD formulated product on the shelves at your local pharmacy, packaged in one of these brands.
But the Australian market is only part of the picture — BOD is thinking global too.
BOD previously had an exclusive distribution arrangement with Health and Happiness Group (H&H, parent company of well known vitamin and supplement brand, Swisse) where BOD was able to generate revenue from around the world.
That exclusivity clause in the deal has now finished, which frees BOD up to supplement its international sales by securing additional global distribution partnerships for CBD products across diverse food and beverage segments.
All in all, BOD is a cannabis focused drug and product development company that is actually generating revenues, and is capped at less than $10M.
In addition to the sales, BOD is progressing R&D and clinical trials in order to deliver premium, scientifically proven and trusted products for the consumer and medical markets — the results of that work could lead to dramatically improved sales and profits.
This all feeds into our Big Bet for BOD...
Our Big Bet
“BOD will deliver a minimum 10x return on successfully commercialising at least one application of its new technology that optimises the human body's absorption of cannabinoids.”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved, and it will require a significant amount of luck. There is no guarantee that it will ever come true.
BOD Science Ltd
ASX:BOD
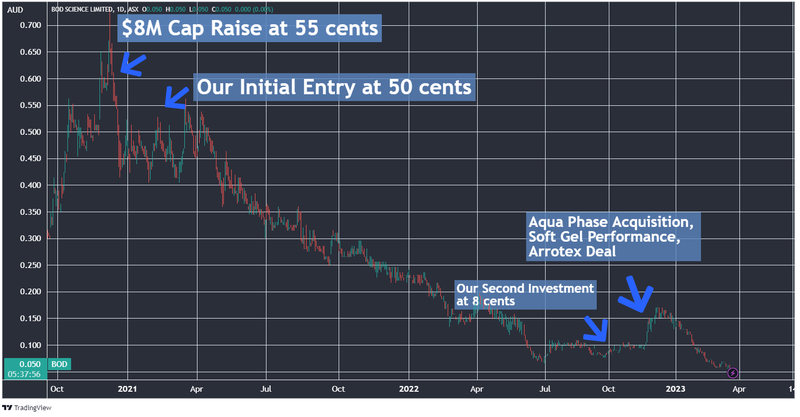
It's been a difficult slog for long term BOD investors like us over recent years.
We first Invested in BOD at 50c in March 2021.
Since then, it's been pretty much all the way down.
We thought BOD’s share price had reached its low point last September, when its market cap dropped below $10M and it announced it was acquiring a transformative delivery format technology, Aqua Phase.
As such, we took an opportunity to average down, and increased our Investment at 8c per share, the same price as the capital raise.
While it looked like BOD had turned the corner after that — it briefly rallied to ~18c in December on the back of progress with two trials — its share price is currently trading down again to around 5c, with a sub-$8M market cap.
We think there could be a few reasons for this, first noting the fairly grim sentiment for the cannabis sector broadly, along with delays from BOD on some key milestones particularly with regards to its clinical trials. BOD also has some acquisition payments to settle, which investors could be waiting for.
The “risk off” mood of investors recently for small cap stocks has also not helped BOD’s plight.
Here is the tale of the tape for the past three years.
That said, the things we liked when we first Invested remain intact: quality management with plenty of skin in the game, and partnerships with big pharmaceutical players that open doors and provide access to big markets.
We are also encouraged by management increasing their share holdings. Management knows the business best, so increasing their investment provides confidence that there is upside ahead, should BOD execute on its plans.
Earlier this month chairman Dr David Baker purchased 764,705 shares on market to take his holding to 4.5 million shares. Dr Baker, alongside fellow board members Jo Patterson (CEO/ founder) and George Livery, also participated in BOD’s previous capital raise in September.
Looking at the balance sheet, BOD held $3.8M in cash at the end of December 2022, including a $1.5M R&D tax rebate. We also note that BOD is debt free.
We will get a closer look at BOD’s financials in the next quarterly report due at the end of April.
BOD has a market cap of less than $8M.
There is potential for a positive share price re-rate should its upcoming trials be successful, in particular the Schedule 3 insomnia treatment.
With such a low market valuation, BOD is leveraged for a positive share price re-rate if its clinical trials prove successful.
How is BOD progressing on the objectives in our Investment Memo?
Let’s take a quick look at the objectives we set for BOD in our Investment Memo published in July 2022.
Specifically, we wanted BOD to deliver on three fronts by the start of 2023 (click on the image for more detail in the full Investment Memo):

To start with, there has certainly been some slippage to our anticipated timelines for two of these objectives, which may have contributed to BOD’s share price fall.
We suspect that the acquisition deal of the Aqua Phase technology in September was also partially responsible for delaying trial progress, given it would have consumed both management focus and resources.
Yet BOD surprised us, making good progress on a couple of trials that we had not focused on — two of which we will go into further detail later. Progressing these trials would also be a factor in causing some schedule slippage with the other trials.
We haven’t heard much regarding Objective #1, the Long Covid trial, which may have taken a back seat to a number of other potential products that could deliver more value for BOD.
Today we will deep dive into Objective #2, the insomnia treatment trials. It appears that this has been delayed by around six months from what we initially expected, but is now on the cusp of commencing.
As for Objective #3 — which relates to the commercialisation of the insomnia treatment — this is where BOD is delivering to expectations.
We still think that BOD has plenty of newsflow ahead, as it is one of the ASX’s most research and development focussed cannabis companies, currently progressing some 11 trials across varying stages.
Success in any one of these could lead to new products and treatments that broaden and/or reach new markets, ultimately driving sales and enhancing its acquisition appeal to bigger companies.
Don’t sleep on this one
As we covered above, we think BOD is in pole position to deliver Australia’s first CBD product available in pharmacies within the next 12 months.
BOD is progressing a Phase IIb clinical trial examining the efficacy of a unique CBD formulation on symptoms associated with insomnia.
BOD last week passed a significant milestone, completing a trial screening of 370 potential patients.
This means BOD should meet its recruitment target of 198 patients by the end of March.
The recruitment and trial was covered in the following news clip on Channel 9 news:
Once all patients are recruited, the eight week trial can start — undertaken by the Woolcock Institute, Australia’s leading sleep research organisation.
The trial is a double blind, randomised and placebo-controlled investigation, utilising both 50mg and 100mg oral CBD doses, in comparison to a placebo.
Upon successful completion, BOD will have sufficient data for an application to register its low dose CBD product with the TGA, which regulates Australian pharmaceuticals and medical treatments.
If approved, BOD can then begin marketing and supplying pharmacies with Australia’s first over the counter CBD product.
This process should be fairly quick and straightforward, by clinical trial standards, with a commercial product potentially being approved within the next 12 months.
And a commercial pathway is already set.
Remember that BOD has already entered an exclusive 5-year licensing and supply agreement for this product.
The agreement is with Arrotex Pharmaceuticals, Australia’s largest supplier of generic drugs to pharmacists, servicing over 5,700 pharmacies nationwide.
Under the terms of the agreement, BOD received an upfront $500,000 cash payment for exclusive supply of the final product to Arrotex, and once the product is on the market, it will start receiving revenues.
It is only very recently that CBD treatments have been made available for purchase at pharmacists.
In February 2021, the TGA down-scheduled low dose CBD for over-the-counter purchase (sales without a prescription are termed Schedule 3). Prior to this, the only channel for CBD treatments was via prescription from an authorised medical practitioner (typically your doctor).
Whilst it is now legal to sell CBD treatments at pharmacies, no CBD drugs have yet been approved for sale in Australia.
Such regulatory change has opened a new market opportunity — our hope is that BOD can be first to capture it.
Research from FreshLeaf analytics is forecasting the Schedule 3 CBD market in Australia to reach A$250M at maturity.
We believe that speed to market will be crucial in capturing and retaining market share of the Schedule 3 market.
Currently, almost 1-in-4 medicinal cannabis patients take a low dose (i.e. under 150 mg) CBD product, according to FreshLeaf.
There is a strong likelihood that once low-dose CBD products become available over-the-counter, many of these patients will prefer to purchase OTC products from pharmacies.
That would translate to a further 10,000 customers nationwide, or ~A$29M p.a. for the Australian market.
Our hope is that BOD can deliver a positive outcome with its clinical trial, paving the way for first mover advantage into this new market and capturing and retaining the lion share unopposed.
CBD skin treatment: another BOD trial we’re watching with interest
Last October, BOD lodged an international patent application for a novel delivery device to combat the skin ageing process using CBD.
The device comprises a novel family of proteins discovered through a research collaboration with the University of Technology Sydney (UTS).
Recent testing showed that treating skin cells with BOD’s delivery device reduces cell death from UV light exposure by up to 30% when compared to untreated cells, and it provides protection against harsh skin oxidants such as hydrogen peroxide.
BOD is now actively advancing licensing opportunities for this product.
We think that skin care products — many of which have dubious or no clinical evidence behind their claims — could benefit from this patented, clinically proven technology.
This is a significant market opportunity. According to Precedence Research, the global anti-aging market is expected to grow at 7.9% (CAGR) from 2022 through to 2030, reaching approximately US$120 billion by 2030.
Given the size of the market, we’re keen to see how this program develops in the year ahead.
Does BOD have a better way to deliver CBD?
Besides the CBD products themselves, BOD is also working on finding the best delivery format for these products.
Most CBD products are now taken as either an oil or tablet. These often have an unpleasant taste and have low bioavailability, that is, the extent a compound becomes completely available to its intended biological destination.
In particular, BOD is progressing two different formats — a soft gel capsule and a water soluble delivery format. Both show very promising results.
Let’s look at the soft gel capsule first.
Last November, BOD reported positive results from a Phase 1 Clinical Trial of its novel CBD softgel delivery format.
That trial evaluated BOD’s ECS BioAbsorb Softgel Capsule against other delivery formats currently available, namely Epidiolex and CBD Oil, across safety, tolerability and pharmacokinetic (PK) profile.
As an aside, Epidiolex — the only FDA-approved CBD drug (you need FDA approval to enter the US market) to treat rare forms of child epilepsy — was acquired by Jazz Pharmaceuticals via its acquisition of GW Pharmaceuticals for US$7BN in 2021.
This gives some idea of just how valuable these drugs can be.
As for BOD’s trial, the soft gel delivery format delivered impressive results.
The study supports the potential for BOD’s unique ECS BioAbsorb Soft Gel to be the benchmark delivery format for this over the counter CBD market, with the trial reporting:
“BOD ECS BioAbsorb Softgel offers over 20% greater bioavailability than Epidyolex and over 400% greater than the CBD Oil solution.”

The maximum concentration of CBD after single oral administration of BOD ECS BioAbsorb Softgel was >60% greater than Epidiolex and >600% greater than CBD Oil solution.
In addition, BOD ECS BioAbsorb Softgel was twice as fast to reach maximum concentration of CBD after a single oral administration of Epidiolex, and five times faster than CBD Oil solution.
So there appears to be potential for this to become the superior way to take CBD compounds. More work is required, but BOD is off to a very good start.
Indeed, this delivery format is the one being used for BOD’s current clinical trial for a Schedule 3 CBD product to treat insomnia. So it may not take that long for this softgel tablet to become available to consumers.
Aqua Phase provides a competitive advantage
The other delivery format BOD is progressing is Aqua Phase, a technology that BOD is acquiring for a total consideration of up to £3M (~A$5.2M) in cash and BOD shares.
Aqua Phase allows for lipophilic compounds, such as CBD, to become soluble (i.e. like salt in water). This would be a first for the CBD industry.
Becoming soluble would drastically improve the bioavailability (i.e. how much is biologically absorbed by the human body) of CBD products, as compared to current oil based methods that tend to be quite poor (6-8% absorption).
The technology offers a CBD product that is not only soluble, but tasteless, colourless, and odourless — traits that we suspect will be of interest to several market makers including beverage brands and over-the-counter consumer CBD products.
By improving the bioavailability, BOD can improve its products through:
- Faster onset
- Better efficacy
- Lower dosing required
- Fewer side effects, and
- Raw material cost savings
We think BOD could gain a competitive advantage by applying the new technology so that existing products deliver enhanced therapeutic impact with less raw material.
The technology could be fairly quickly applied to BOD’s current and upcoming suite of products, including its existing medical cannabis and over-the-counter insomnia treatment about to be trialled.
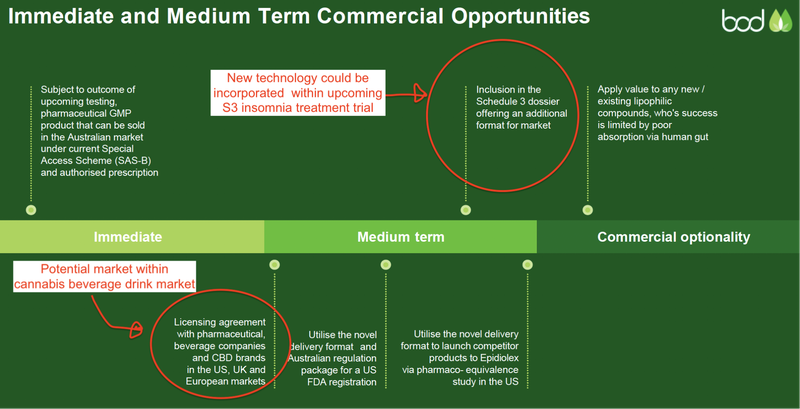
Below is a useful video that demonstrates how Aqua Phase works.

Only the completion of the Pharmacokinetic (PK) Study remains to trigger the initial acquisition consideration of £1M (~A$1.7M).
This study tests how the body handles CBD products administered via Aqua Phase compounds, essentially investigating the extent that the technology improves the bioavailability of CBD products.
Due to third party delays in the PK study, the closing date to finalise the acquisition has been extended by three months from the end of March to the end of June 2023.
The remaining £2M is due once two subsequent milestones are delivered over the next 12-36 months. BOD can elect to pay this in scrip (BOD shares) or in cash.
What’s next for BOD?
With ethics approval already granted for the CBD-based insomnia treatment clinical trial and patient recruitment almost complete, we’re keen to see the eight week clinical trial follow quickly.
On this timeline, we anticipate BOD submitting its dossier to the TGA sometime around July this year. The company indicated that this is being progressed in parallel to the clinical trial.
Subject to successful outcomes of the trial, it’s feasible that BOD could have its CBD treatment for insomnia available in pharmacies by late-2023, or in the first half of 2024, positioning it as one of the first, if not the first, to offer CBD products over the counter.
BOD’s Investment Memo
Below is our Investment Memo for BOD where you can find a short, high level summary of our reasons for Investing.
The ultimate purpose of the memo is to record our current thinking as a benchmark to assess the company's performance against our expectations for the following 12 months.
In our BOD Investment Memo, you’ll find:
- Key Objectives for BOD for the coming year
- Why we are Invested in BOD
- The key risks to our Investment Thesis
- Our Investment plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

