DXB Recruits First Patient, Gets FDA IND Approval
Disclosure: The authors of this article and owners of Next Investors, S3 Consortium Pty Ltd, and associated entities, own 2,825,000 DXB shares and 625,000 DXB Options at the time of publication. S3 Consortium Pty Ltd has been engaged by DXB to share our commentary and opinion on the progress of our investment in DXB over time.
Our 2021 Biotech Pick of the Year, Dimerix (ASX:DXB), yesterday recruited its first patient for its Phase 3 FSGS trial - a big step forward towards our #1 Objective for the company.
This means the clinical trial is actually happening, right now. This is exactly what we Invested in DXB for when we initiated coverage at 20c in August 2021.
The commencement of trials is a major milestone for DXB - and the company put in a lot of legwork to get to this point.
Part of that legwork included getting ethics/regulatory approval in 12 key jurisdictions including, most recently, the US - an important market for DXB’s treatment.
The US is a key market for DXB’s treatment as a large portion of patients reside there.
Focal segmental glomerulosclerosis, or more simply “FSGS”, is characterised by a dysfunction in the part of the kidney that filters blood.
FSGS has an outsized impact on the US healthcare system because people who suffer from FSGS usually require a kidney transplant. This is an expensive option and not available to everyone.
With the pressing need for a treatment, especially in the US, we think DXB should garner attention from investors ahead of the interim trial results in mid-2023.
Whatever happens in the broader market, the next 12 months are critical for DXB, as the Phase III clinical trial is the last major step before commercialisation.
Most biotech companies don't even get to Phase III, but as each biotech passes through trial phases, the chance of a successful outcome improves.
This is called "phase transition success", or the likelihood of DXB getting through Phase 3 and into the New Drug Application stage. (In the US, that figure is around 58% as a cohort, this isn’t specific to DXB).
The final stage before going into the market is the New Drug Application phase and around 90% of treatments make it past this hurdle - meaning regardless of the specific merits of DXB’s drug, we think it should have a good shot from here.
DXB is also sitting well below our entry point and we think that’s largely due to market factors.
We initially Invested in DXB at 20c in August 2021 and the share price has bounced around since then. It is currently sitting at ~14.5 cents - 28% below our entry point.
DXB isn’t alone here, biotechs across the board have been hammered over the last four months as funds reposition their capital in a market facing inflation and rate hikes.
The good news is that DXB is fully funded through to the interim trial results of its Phase 3 FSGS trial, with ~$16.8 cash at bank as at 30 March 2022.
This means that DXB can weather the current storm affecting the broader biotech market, and come out the other side in good shape (depending on trial results of course).
DXB’s CEO Nina Webster will be presenting at Monsoon’s Twilight Investor Briefing via zoom today at 4:30PM (AEST) to go over yesterday’s development. We will be attending and you can register to attend here:
REGISTER: DXB’s Investor Webinar, today at 4:30PM (AEST)
Today we’ll discuss the following:
- What does the first patient recruited mean?
- What does US FDA IND approval mean? And why this is important
- Map out what milestones we’re looking for as the Phase 3 trial progresses.
What does the first patient recruited mean?
For biotech companies, the first patient recruited into a clinical trial is a major milestone.
It marks the beginning of the ‘recruitment and data collection phase’ of the study, leading up to the trial results - the major share-price catalyst that investors look forward to.
With the majority of sites actively recruiting patients, we expect DXB’s recruitment numbers to accelerate over the next few months.
The magic number of patients recruited for DXB is 72 - the cohort number required for the first interim trial results.
Now that the first patient has been recruited he or she will be dosed within the next six weeks, as seen below:
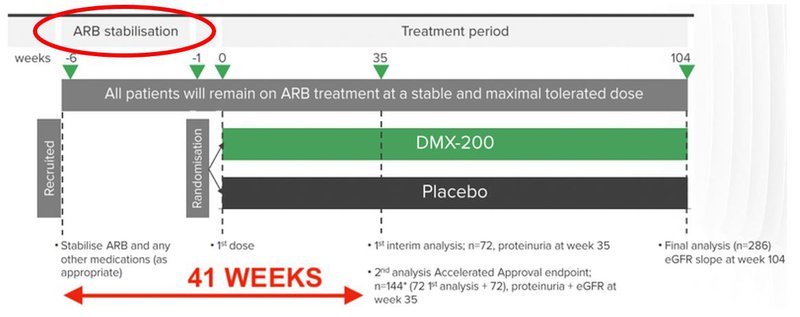
That circle in the top left is the ARB stabilisation period - ARB stands for angiotensin II receptor blocker and is the current standard of care for FSGS.
The study is aiming to see if DXB’s treatment is better than the current standard of care (ARB) at treating FSGS.
In order to remove as many variables as possible, patients must be on the ARB for six weeks prior to the start of the treatment. As ARB is the current standard of care, this period may not be relevant for some patients.
As mentioned, 72 patients is the cohort required for the interim analysis.
Reaching that figure is the announcement that we’ll be waiting for. Smaller chunks of patient recruitment progress will come in the form of investor presentations, quarterly reports and other announcements to the market.
From the 72nd patient recruited, the trial will take up to 41 weeks to get to a first interim analysis. DXB will continue to recruit patients throughout this period, in anticipation of the second interim analysis point.
Why is the interim analysis so important for DXB?
DXB’s treatment has ‘Orphan Drug Designation’ in the US and UK, a designation which is reserved for ‘rare diseases’.
This designation means that marketing approval for DXB’s treatment can be fast tracked, as well as receive tax and other concessions (such as the 7 year exclusivity arrangement) to help it get to market.
There are two interim analysis points in the trial, the first interim analysis point (72 patients) gives investors a good indication of the progress of the trial, and the second interim analysis point (144 patients) “may enable accelerated marketing approval” according to DXB.
This means investors don’t need to wait for the final analysis data before DXB’s treatment can be approved for marketing in the US and UK, that is, IF the trial is successful.
Yesterday’s announcement progresses our #1 Objective for DXB, as it is now truly in the ‘recruitment and data collection phase’ of the trial:

What are the US FDA IND key approvals? And why are they important?
Three weeks ago, DXB secured FDA Investigational New Drug (IND) approval in the US for its Phase III clinical trials for FSGS.
The FDA approval for DXB’s Phase III study was made under the Investigational New Drug (IND) regime in the US - a key market for DXB to develop.
That was the last of 12 jurisdictions it was seeking approval in so DXB now has a clear run at proving its treatment for FSGS is both effective and safe.

Securing approvals in the US was important because over one-third of all FSGS patients are located there.
FSGS affects 210,000 people around the world and there is no treatment currently in the market.
There are roughly ~40,500 FSGS patients in the US currently, and the addressable market globally comes to around $1B.
Again, we note that DXB is well funded, with ~$16.8 as at 30 March 2022, and will continue patient recruitment for its Phase III clinical trial on FSGS.
Securing this FDA IND approval is another major milestone for the company.
What’s next for DXB?
Yesterday’s development progresses our #1 Objective for DXB and a major milestone for the company.
So far DXB has ticked off a number of milestones on the way towards a key potential catalyst - announcing the interim study results. A positive outcome could lead to the fast-tracked commercialisation of DXB’s FSGS treatment.
All of the groundwork has now been set up for the first study period for the Phase III clinical trial, and we will keep an eye out for the first patient dosed in the trial as well as patient recruitment updates along the journey.
We filled our DXB Investment Memo with the following milestones for Objective #1 as a result:
✅ Phase III Study Design Complete
✅ Study Design approved by the FDA
✅ Ethics approvals complete 12/12 countries
🔄 Site Setup (assumed 80% complete)
✅ First Patient Recruited
🔲 Patient Recruitment Updates
🔲 72 Patients Recruited
🔲 Patient Recruitment Updates
🔲 Interim Results Announced
The key patient recruitment update will come once 72 patients are recruited. From there, it will be 41 weeks before the interim results are announced.
Recruitment is currently underway for DXB and we expect the company to meet its anticipated timeframe for announcing results by the middle of 2023.
Site setup is also important as this means DXB has formalised all arrangements for the clinical trials at the various sites around the world (training, supplies etc.)
That said, given the rare nature of the disease, recruitment can be delayed. This is one of the key risks we identified for DXB:

While we think the 12 jurisdictions that have approved the trial helps and de-risks our DXB Investment, there could still be delays in recruitment.
Our DXB Investment Memo
In our DXB Investment Memo you’ll find:
- Key objectives for DXB in 2022
- Why we invested in DXB
- What the key risks to our investment thesis are
- Our investment plan
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






