TRI with Significant Update on their Phase 2 Clinical Trial
Yesterday, our mental health technology Investment TrivarX Limited (ASX: TRI) has provided a significant update on its Phase 2 clinical trial (SAMDE study) for the validation of its proprietary AI-backed algorithm, MEB-001.
TRI said yesterday that the company has successfully enrolled 362 out of the targeted 400 patients in the study, which aims to evaluate the effectiveness of MEB-001 in screening and diagnosing major depressive episodes.
The completion of the Phase 2 SAMDE study is expected in the next few weeks, with results to be announced shortly after.
One of the main reasons we Invested in TrivarX is their use of AI before it was “popular.”
TRI’s Head of AI, Dr Massimiliano Grassi, has been working with AI for approximately 6 years. TRI’s algorithm (MEB-001) has advanced to Phase 2 trials and is getting better as time passes and more data is collected.
In preparation for the potential commercialisation of MEB-001, TrivarX has appointed W. Drew Palin, M.D., a very experienced healthcare executive with over 30 years of experience in the medical technology sector, as a strategic advisor.
Dr. Palin's expertise will be invaluable in advancing various commercial opportunities, such as partnerships, licensing agreements, and other commercialisation initiatives.
How does this impact our Investment Thesis for TRI?
Positive outcomes from this trial will further support TrivarX's ongoing engagement with the United States Food and Drug Administration (FDA) for regulatory approval of MEB-001 through the De Novo pathway.
We see this news as relating to the following key reason we Invested in TRI:
Imminent Phase 2 clinical trial results
400 patients' sleep data is being screened for depression using TRI. IF the trial result is positive, it could open the door to US FDA regulatory approvals and potentially even training the AI algorithm to screen for other types of mental health issues too. The final results are expected in the next couple of months. We think they will be a catalyst for TRI’s share price.
Yesterday’s news furthers Objective #1 from our TRI Investment Memo:
Objective #1: Complete Phase 2 clinical trial, release results
TRI is currently conducting a Phase 2 trial in the US on its algorithm for detecting current Major Depressive Episode (cMDE). Final results are expected in the June quarter (before July 1st 2024).
And yesterday’s patient recruitment update as reducing the following risk:
Clinical trial risk
Clinical trial outcomes are never certain - TRI might not be able to generate results good enough for the regulator to approve the product. Clinical trials, even low cost ones, require capital in order to be conducted. A clinical validation study, which TRI may also have to complete could cost additional money.
Click here to read our TRI Investment Memo in full
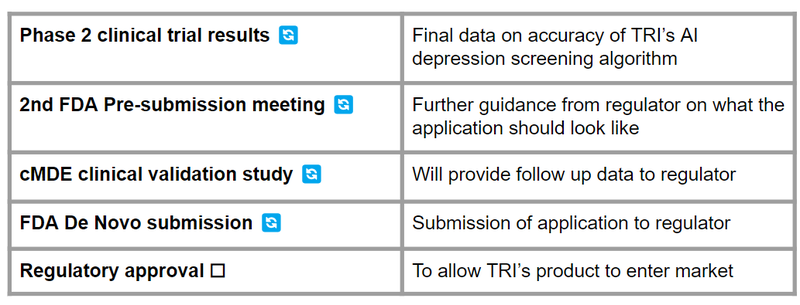
What’s Next for TrivarX?
In the end, we want to see approval from the FDA, and here are the next steps needed from TRI for it:
Read more about TRI in our first note on our Investment in the company:







