TRI reports clinical trial results for current major depressive episode (cMDE)
Our Investment TrivarX (ASX: TRI) just released results from trials screening for current major depressive episode (cMDE) in US Veterans.
(The trial was conducted in collaboration with the Greater Los Angeles Research and Education Foundation and Veterans Affairs Greater Los Angeles Healthcare System)
TRI already has an algorithm which it has shown can accurately detect what is called a “current Major Depressive Episode (cMDE)” from a person's sleep data.
TRI’s previous trial data was generated from sleep clinics, where brain waves (EEG) and heart rate and heart rate variability data (ECG) were measured with devices that look like this:
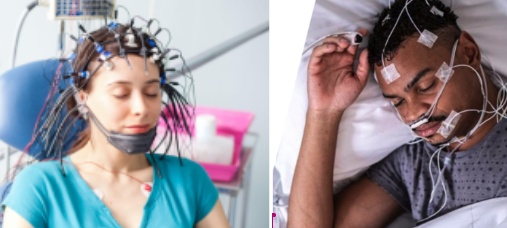
In this new clinical trial, TRI used a “single lead ECG” to (No massive helmets in a sleep clinic, no multiple strips of pads connected to the chest).
In addition to using the single-lead ECG signal, patients also wore a wrist-worn wearable device during the night - providing the company with additional data for further R&D and commercialisation.
(basically TRI’s first look at how its tech would work in a mass market device like a “wearable” where it is even easier to collect data - and used by hundreds of millions of people)

TRI released results from 57 of the 60 patients enrolled in the clinical trial and showed:
Results from this included:
- Single-lead algorithm sensitivity: 97%
(Basically, TRI’s tech was able to detect 97% of cases objectively in patients who may otherwise remain undiagnosed using traditional screening approaches).
- Single-lead algorithm specificity: 64%
(Basically, TRI’s tech was able to correctly identify 64% of patients who did not have current major depressive episode (cMDE)).
Compared to the existing results from its algorithm (MEB-001) which showed:
- Sensitivity: 88%
- Specificity: 68%
We think the results today look relatively strong - especially considering the sensitivity improvements relative to TRI’s existing algorithm.
Next, we want to see what TRI plans to do with this data - whether it be a larger trial or maybe some sort of development deal that gets the tech built into single lead ECG devices…
While we wait for that we are looking forward to seeing TRI close out its most recent acquisition.
What’s next for TRI?
🔄 Finalising the acquisition of the intellectual property from Nucleics
The main next step will be to see TRI finalise the acquisition of Nucleics.
After that it will be all about the work leading up to clinical trials in CY2026.
TRI mentioned that work would start on manufacturing and quality control for stable imaging compounds as well as FDA engagement for a Phase 1 clinical trial scheduled for CY26.
So we won't have to wait years in the pre-clinical setting before we see the tech being tested…
Here are the milestones we will be tracking:
🔲 Manufacturing and quality-control validation for stable isotope compounds
🔲 Engaging in regulatory pre-submission activities in key markets (US and EU)
🔲 Trial design and preparations for a Phase 1 clinical trial scheduled to commence in CY26






