TRI acquires early brain tumour imaging technology
Our Investment TrivarX (ASX: TRI) has bought in a new asset in the neuro-oncology space…
Yesterday, TRI announced the acquisition of “Stabl-Im” - a novel brain imaging technology for early and safe detection of brain tumors.
The acquisition is from Nucleics - whose founder and CEO is Dr Daniel Tillett.
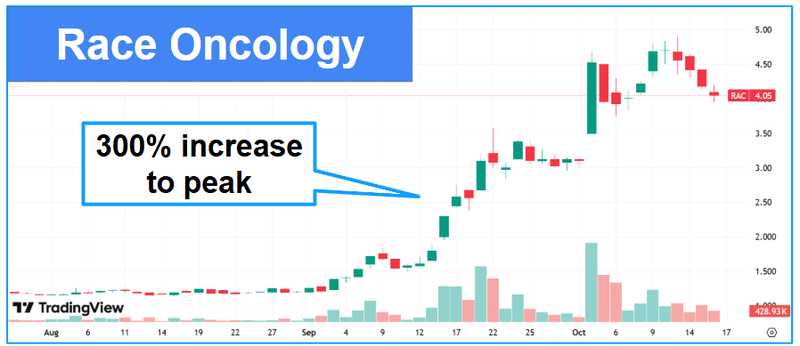
Tilllet is also the CEO & Managing Director of Race Oncology Limited (ASX: RAC) which is currently capped at $750M - Race is in the oncology treatment space.
Dr Tillet also personally cornerstoned the raise for $500K (of a total $4.2M raised at 0.8c).
Race’s share price is up ~300% in the last 3 months alone:
(Source)
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
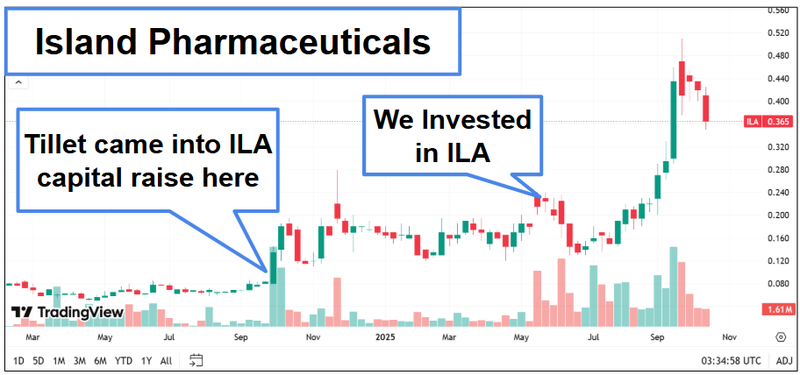
We have also invested alongside Dr Tillet in Island Pharmaceuticals (ASX: ILA) where he is a major shareholder (owning 5.55% of the company) too.
ILA is up 147% from our Initial Entry Price.
(Source)
More on Nucleic’s - what is TRI acquiring?
At a very high level, TRI is acquiring IP that could potentially diagnose brain cancer, early and without invasive imaging/surgeries.
The IP being acquired could label and identify replicating cells in the brain (which is something that doesn't happen when an adult is healthy and has no brain tumours).
IF TRI can prove the technology works then it could potentially mean brain cancers are able to be imaged/monitored using standard MRI…
(as opposed to things like CT scans, lumbar punctures (where cerebrospinal fluid is taken for analysis of cancer cells) or things like a biopsy where there is the need for invasive surgery.
TRI’s tech could also work alongside all of the existing diagnostic process’ solving for early diagnosis shortfalls of something like MRI (which can only only detect tumours larger than 2-3mm),
Brain cancer is one of those diseases where early diagnosis and treatment can be a game changer so any role TRI’s tech plays in the space could be valuable from a clinical perspective.
For context on the value of something that works in this space - TRI put up the following numbers for the size of the Neuro-oncology diagnostic and treatment markets:

(Source)
What is TRI paying for the new assets?
TRI will pay a $250k fee and be given a 90 day exclusive option to acquire the IP.
IF the option gets exercised, TRI doesn't have to pay anything upfront, instead it issues the vendors two sets of performance rights that convert based on the following milestones:
- 250M Class A Performance shares that will vest on the completion of phase I trials (within 4 years of the deal being completed)
- 500M Class B Performance shares that will vest on the completion of phase II trials (within 4 years of the deal being completed)
We like that the transaction is structured with a low upfront cost to TRI and long dated performance shares that only vest on the assets delivering good news…
TRI raised $4.2M to go with the deal at 0.8c.
The capital raise will get settled across two tranches - the first tranche for $700K settling on the 27th of this month and the remaining $3.5M subject to shareholder approvals in December.
What’s next for the tech being acquired?
🔄 Finalising the acquisition of the intellectual property from Nucleics
The main next step will be to see TRI finalise the acquisition of Nucleics.
After that it will be all about the work leading up to clinical trials in CY2026.
TRI mentioned that work would start on manufacturing and quality control for stable imaging compounds as well as FDA engagement for a Phase 1 clinical trial scheduled for CY26.
So we won't have to wait years in the pre-clinical setting before we see the tech being tested…
Here are the milestones we will be tracking:
🔲 Manufacturing and quality-control validation for stable isotope compounds
🔲 Engaging in regulatory pre-submission activities in key markets (US and EU)
🔲 Trial design and preparations for a Phase 1 clinical trial scheduled to commence in CY26
Dr Tillet will be a part of the team leading the first phase of work on the tech:

(Source)






