NTI in the news for Rett Syndrome treatment, new CEO to come
Today, our biotech Investment Neurotech International (ASX: NTI) announced a new MD/CEO will be taking the helm in February next year.
We’re keen to see what Dr Anthony Filippis can achieve with NTI - which has delivered a string of positive clinical trial results since we Invested in September 2023.
(More on this appointment in a moment)
But the main news today, is the actual news - our Investment NTI made an appearance in a story on Channel 7 last night:

(Click here to watch the full story)
NTI featured on Channel 7 News Australia with its groundbreaking clinical trial results from the Company's Phase I/II Rett Syndrome in a story which profiled one of the patients who participated in the trial, along with Professor Carolyn Ellaway - Principal Investigator of the clinical trial.
NTI’s treatment for Autism Spectrum Disorder and upcoming Cerebral Palsy trials also got a mention - which remain key reasons we remain Invested in NTI.
Rett Syndrome is a rare genetic neurological and developmental disorder that affects the way the brain develops in very young women.
Rett Syndrome symptoms are horrible and permanently life altering for both the patient and caregivers.
But we think NTI’s biopharmaceutical NTI164 offers hope for patients and caregivers with its sustained clinical trial performance which resulted in the trial being extended out to a 52 week period.
Below are the results from NTI’s clinical trial on Rett Syndrome which show sustained benefits out to a 20 week timeframe:
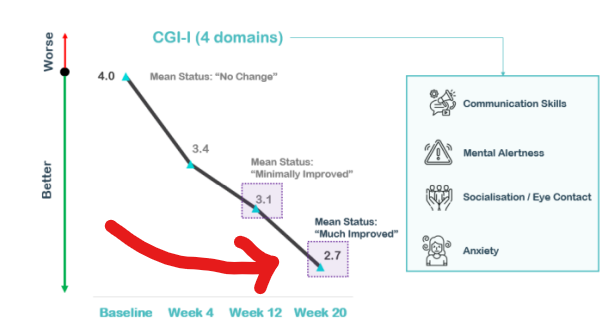
(Source)
Read more about NTI’s strong clinical trial results for Rett Syndrome below:

NTI’s Phase I/II Rett Syndrome clinical trial results improve upon $2.4BN capped Neuren’s results…
As for the incoming NTI MD/CEO, Dr Anthony Filippis, he’ll be taking charge at a critical moment for the company after departing CEO Tom Duthy helped deliver a string of positive clinical trial results.
Dr Filippis is an experienced dealmaker, who has raised capital and closed many deals, including with blue-chip companies Siemens Healthineers, AstraZeneca, Schering Plough (acquired by Merck), and Meditech (acquired by Alchemia).
Welcome to NTI Dr Filippis:

(Source)
What’s next for NTI?
We’re particularly keen to see what impact the new NTI MD/CEO can have across commercialisation when he takes the helm on 1 February 2025:
Objective #2: Secure a global partner for NTI164
We want to see NTI advance commercialisation initiatives and, ideally, accelerate them. This could be a source of additional capital for the business to fund offshore trials and advance regulatory requirements.
Milestones
🔲Licensing/partnership agreement
🔲Partnership on global clinical strategy
🔲Potential M&A deal (Strategic equity investment?)
Source: NTI Investment Memo 14 August 2024
Read more about NTI’s strategy to commercialise it treatment in the article below:







