NTI completes autism trial recruitment, two catalyst early next
We were pleasantly surprised this morning to learn that our biotech Investment, Neurotech International (ASX: NTI) had successfully completed recruitment for its autism trial.
Patient recruitment for clinical trials doesn’t always go according to plan - so today’s NTI announcement was a good one.
It means NTI is on track to deliver results in as little as three months.
So we won’t have to wait long for our next NTI catalyst - in fact there are two catalysts due to arrive in Q1 2024 (Phase I/II Rett Syndrome clinical trial results + Phase II/III ASD clinical trial results).
While Phase I/II Rett Syndrome trial is for a niche, orphan disease, the Phase II/III ASD is for a large Total Addressable Market (TAM).
We think both trial results to come in Q1 2024 provide NTI with good looks at kicking big goals.
Earlier results from the PANDAS trial were profoundly positive, with the trial meeting the primary endpoints.
In addition, we think the outlook for more positive results is strong, after NTI previously released data on the safety and efficacy of its NTI164 biopharmaceutical from its Phase I/II ASD trial.
Combined with the PANDAS trial results, we think this further built the evidence base for the NTI164’s anti-neuroinflammatory properties.
More on NTI’s Phase II/III ASD clinical trial…
The Phase II/III ASD trial has now recruited 56 patients with Level 2 (requiring substantial support) and Level 3 (requiring very substantial support) autism (ASD).
That first trial, which was a Phase I/II trial, was extended.
Which we think implies that those carers with children on the trial have seen a marked improvement in their children’s symptoms and are comfortable with the safety of NTI’s treatment.
Likewise, the trial design of the current Phase II/III trial allows for an extension period of an additional 38 weeks, if the patients choose to continue taking NTI164.
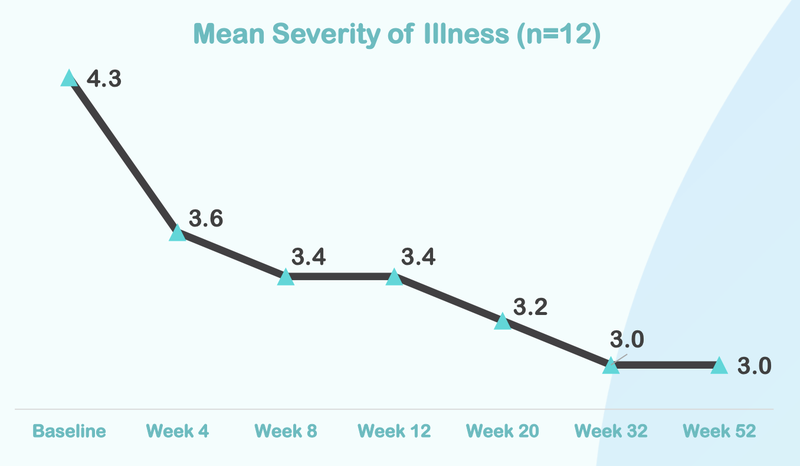
Below is the data from NTI’s first ASD trial…
From this chart you can see that the severity of illness for children with Autism moved from “Markedly” to “Mild”.
Here is how the treatment reduces over time, with significant improvement after just four weeks of taking the treatment:
ASD makes up roughly $6.1BN of the NDIS total $35.5BN expenditure in 2022 and equates to an implied per year expenditure of $32K per NDIS participant that has ASD.
Autism Spectrum Disorder (ASD) is estimated to affect around 1 in 100 children around the world, and in Australia, 34% of the 550,000 National Disability Insurance Scheme (NDIS) participants have ASD, and 40% are 14 years old or younger.
If NTI’s treatment is proven safe and effective for ASD, we think it could become an important part of the overall care for ASD sufferers, ease the burden on both carers and government programs, and as a result, make NTI’s treatment commercially attractive.
We see NTI’s ASD trial results as potentially opening up a large Total Addressable Market (TAM) commercialisation opportunity in Australia - with the rest of the world, in particular the lucrative US healthcare market to follow subsequently.
ASD - especially the more severe forms, presents a big challenge for carers, healthcare systems and most of all, those who actually suffer from it.
We’re hoping NTI’s Phase II/III trial results in Q12024 can help build the evidence base for a long-term solution for ASD and ideally, some major commercialisation opportunities.
What’s next for NTI?
Catalyst #2: 🔄 Phase I/II Rett Syndrome clinical trial (results in Q1 CY2024)
A trial on another child neuropsychiatric disorder called Rett Syndrome - Neuren Pharmaceuticals re-rated ~1300% on commercialisation of its treatment for this disorder. We think NTI could be safer than Neuren’s treatment and if it works better or similar, hopefully, re-rate accordingly.
Catalyst #3:🔄 Phase II/III trial for Autism Spectrum Disorder (results in Q1 CY2024)
After promising Phase I/II trial results, we see this trial as potentially opening more doors when it comes to commercialisation.






