NTI Autism results get even better out to 12 weeks
Neurotech International (ASX :NTI) just reported that further analysis of its Phase II/III autism trial showed patients receiving NTI164 experienced significant additional improvements from week 8 to week 12.
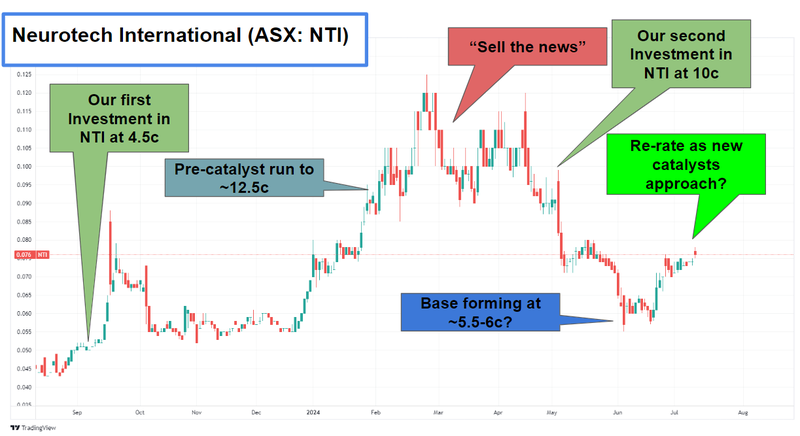
And the share price looks to finally be responding to good news…
We’re quite happy with our NTI Investment in terms of operational performance and trial results, as the company continues to execute.
But we’re a bit confused as to why NTI’s share price hasn’t moved higher up the charts - as NTI has delivered multiple successful clinical trials
We were surprised with the selling on the news (in February to April) and are hoping NTI’s cap structure has stabilised now and can finally start re-rating upwards.
We’ve noticed the NTI share price starting to bounce back off a potential base in the last month:
And the good news just keeps rolling in…
NTI reported that further analysis of its Phase II/III autism trial showed patients receiving NTI164 experienced significant additional improvements from week 8 to week 12.
Patients on NTI164 saw a 56% reduction in symptoms from baseline to week 12, with symptoms “barely noticeable” at the 12 week mark:
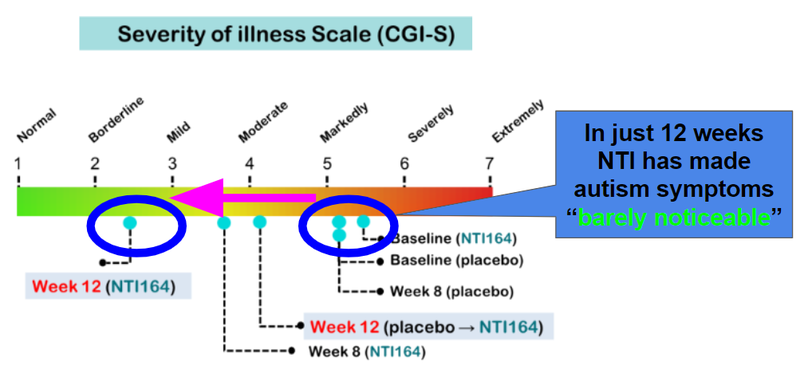
Other important aspects of today’s announcement:
- For patients receiving NTI164 (n=26), there was a 36% improvement in symptoms from week 8 to week 12 as measured by the Clinical Global Impression - Severity of illness Scale (CGI-S)
- Patients who crossed over from placebo to NTI164 at week 8 showed an immediate 21% improvement in symptoms after just 4 weeks on the drug:
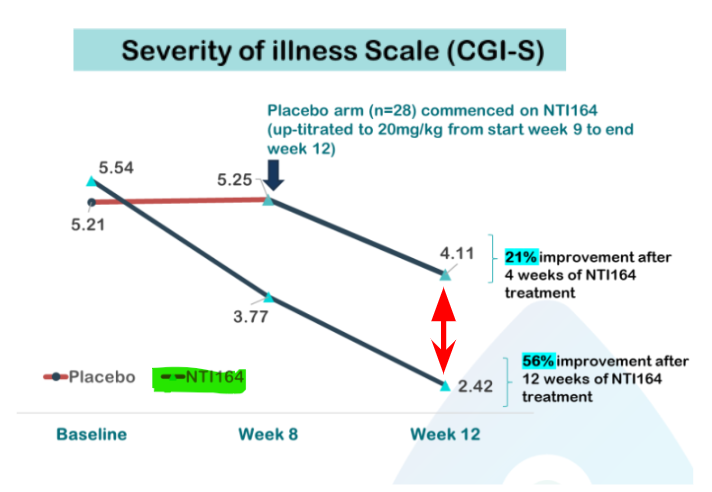
There are currently no approved treatments available to ASD patients - meaning today’s results from NTI should only improve NTI’s case to regulators going forward.
In a nutshell, we think these extended results from the Phase II/III autism trial are highly encouraging and further validate NTI164 (NTI’s biopharmaceutical) as a potential breakthrough treatment for autism spectrum disorder (ASD).
The significant symptom improvement at 12 weeks was also a key win for us as NTI Investors.
If NTI’s ASD successfully makes it to market - we believe it could be life-changing for ASD patients and their families.
The rapid 21% improvement in just 4 weeks for patients crossing over from placebo also highlights the potency of NTI164's effects.
With 1 in 100 children affected by ASD, a safe and effective treatment like NTI164 could fill a major unmet need.
With no serious adverse events, and no adverse events attributable to NTI’s treatment, we think things are looking good for NTI after the company completed its Phase II/III trial for ASD as well as its Phase I/II Rett Syndrome trial (which also met its primary endpoint, which we can’t forget about either):

NTI’s two clinical trial results are in - Treatment success for BOTH Autism AND for Rett Syndrome
How does today’s NTI news impact our Investment Memo?
Promising efficacy data at treating Autism, large addressable market
If NTI’s treatment is proven safe and effective for Autism Spectrum Disorder (ASD), we think it could become an important part of the overall care for ASD sufferers, which is 1 in 100 children.
Source: NTI Investment Memo 18 September 2023
We think today’s NTI results will only strengthen the company’s case to regulators, potentially opening a very large addressable market, to complement the company’s more niche indications such as Rett Syndrome and PANDAS/PANs which NTI has just submitted its Orphan Drug Designation application for.
Clinical trial risk
It is important to be aware that clinical trials can be unsuccessful.
Source: NTI Investment Memo 18 September 2023
Todays news was another positive towards mitigating this risk in the ASD treatment.
What’s next for NTI?
- FDA response on Orphan Drug Designation expected in ~3 months
- Advancing NTI164 through clinical pipeline across multiple rare neurological disorder indications (including Cerebral Palsy)






