IIQ shows promising results in aggressive Breast Cancer treatment
Our biotech Investment Inoviq (ASX: IIQ) just released data for its Triple Negative Breast Cancer therapeutic.
IIQ is a cancer diagnostics and therapeutics company developing an exosome platform technology.
Exosomes are tiny, microscopic extra-cellular “containers” released by ALL human cells.
Exosomes can carry ‘messages’ - clues as to whether or not someone has a disease - which is why IIQ has applied the platform tech to develop diagnostics products.
IIQ is also developing therapeutics to see if these "messengers" can be “weaponised” to locate and kill cancer cells.
Yesterday’s data was from IIQ’s CAR-exosome therapy being developed in partnership with the Peter MacCallum Cancer Centre.
With its therapeutic, IIQ’s first target is Triple Negative Breast Cancer (TNBC) - which currently has no approved cell therapies treating it.
(The main way of treating it at the moment is chemotherapy)
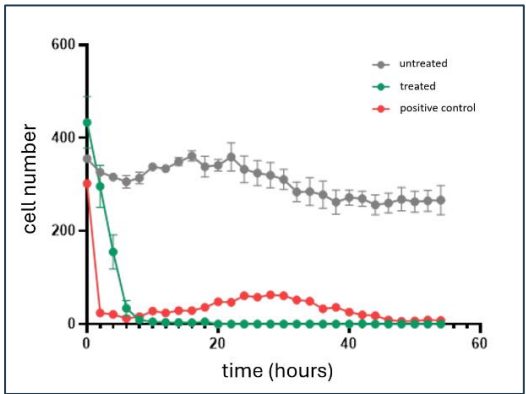
Yesterday IIQ confirmed that its therapeutic was able to kill more than 90% of the cancer cells within 10 hours of treatment.
IIQ also announced that cells that were left untreated or given a different type of exosome as a control group showed barely any (if any) tumour reduction.
These results were from “in-vitro” (in test tube) studies.
Next, IIQ plans to test its therapeutic in animals - starting next quarter (Q4-2025).
(Source)
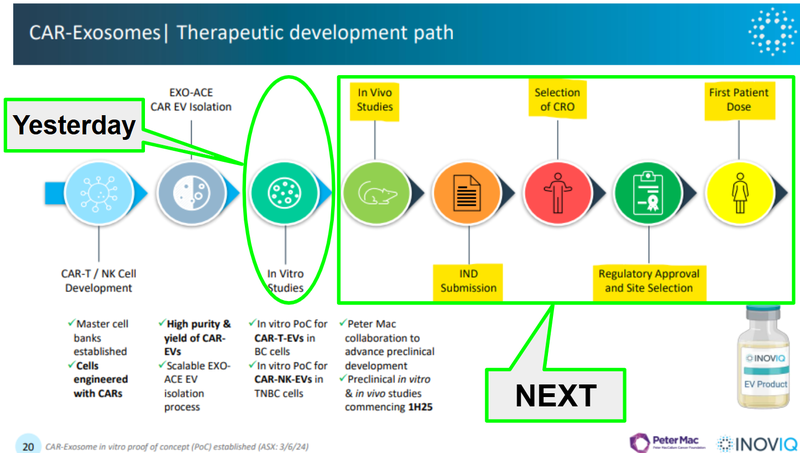
Here is a slide we liked from a previous IIQ webinar which showed the development process for IIQ’s solid tumour therapeutic:
(Source)
Another key takeaways from yesterday’s announcement
We also noticed the following in yesterday’s announcement from Professor Phillip K Darcy:
(Phil is the group leader of the Cancer Immunotherapy Laboratory at the Peter MacCallum Cancer Centre and NHMRC Principal Research Fellow… and is on IIQ’s advisory board)

(Source)
Professor Darcy is basically commenting on how IIQ’s therapeutics could end up becoming the next generation of cell therapies - with specific mention of the “safety and efficacy” advantages over existing cell therapy treatments on solid tumors.
(Hear what Professor Darcy thinks of IIQ’s platform tech and how it will be applied to solid tumours from 21:17 until about the ~26 minute mark).
This safety/efficacy advantage was explained very well by Professor Gregory Rice (IIQ’s Chief Scientific Officer) in the same webinar webinar from earlier in the year.
After listening to both of them run through how IIQ’s tech is different to existing cell therapies we are really looking forward to the animal data in Q4…
What else is IIQ working on?
We mentioned earlier IIQ was also focussed on developing cancer diagnostics products…
IIQ’s most advanced diagnostic is for ovarian cancer.
IIQ announced the results from the test back in June and showed its test detected all stage 1 and 2 ovarian cancers - with no missed diagnosis.
And importantly IIQ exceeded globally accepted clinical performance criteria for ovarian cancer screening in asymptomatic women (women that show no symptoms).

We still like this part of IIQ’s business.
At the moment there are no ovarian cancer screening tests that are approved by regulators - due to their poor early-stage detection of cancer.
And we think IIQ has the opportunity to develop the first-ever population screening tool for ovarian cancer.
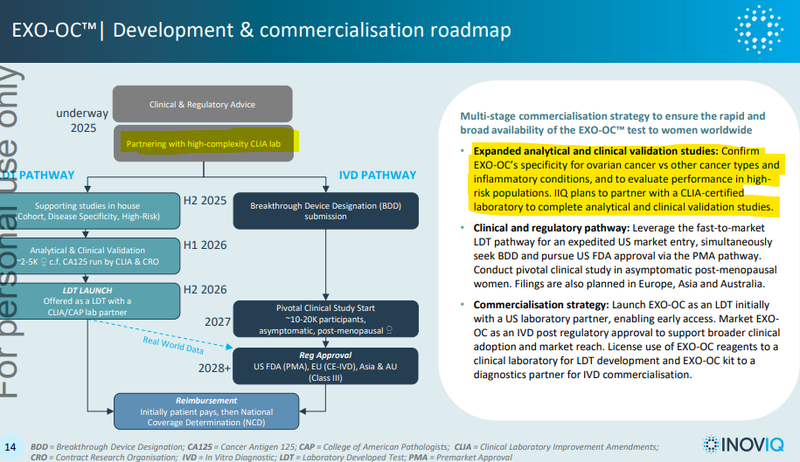
Next for this is IIQ finding a partner to run clinical validation studies:
What’s next for IIQ?
Here’s the rundown of future catalysts from a recent IIQ presentation:

In particular we are looking forward to the following catalysts:
- 🔄Updates on solid tumour therapy in vivo studies (targeting Q4 of this year for in vivo results with Peter Mac)
- 🔄A lab partner for SubB2M tests (for breast cancer monitoring)
- 🔄Clinical validation studies for highly accurate ovarian cancer screening test
- 🔄Development of exosome diagnostic for neurodegenerative conditions like Alzheimer’s and Parkinson's.