IIQ shows 100% survival rate and tumour reduction for Triple Negative Breast Cancer in mice models
Our biotech Investment Inoviq (ASX: IIQ) just showed that its solid tumour therapeutic (CAR-NK-EV) for triple negative breast cancer was able to achieve:
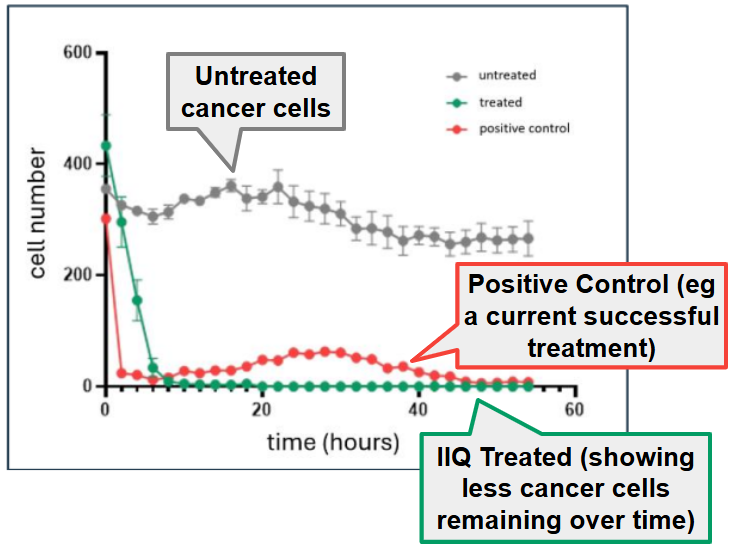
- 61.5% tumour reduction over 28 days - outperforming unmodified NK-EVs
- A 100% Survival rate in all mice treated with IIQ’s therapeutic (compared to 66.7% for the control group). AND
- NO safety concerns (no adverse side effects from taking the therapeutic).
Today’s data came from “in vivo” trials, which is basically when a biotech company tests its products in animals (mice models).
Basically, it's the second stage of “proving a concept” before a company can start thinking about human clinical trials.
Today’s data follows earlier “in vitro” data (in test tubes) which showed that IIQ’s therapeutic was able to kill more than 90% of the cancer cells within 10 hours of treatment.
(Source)
Now IIQ’s shown its therapeutic works in lab studies and mice models - next IIQ can start preparing for human clinical trials - which IIQ says will be in “2028”.

(Source)
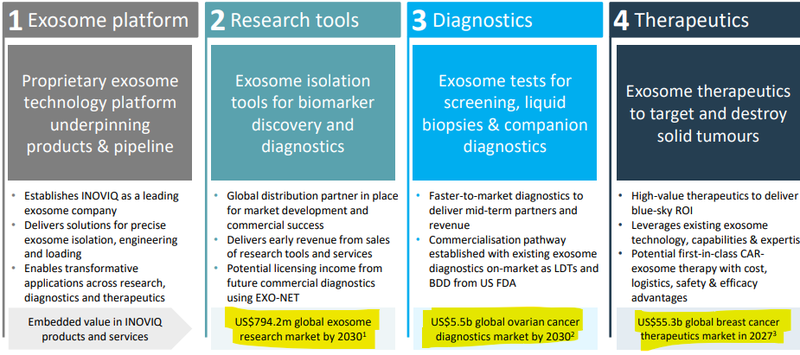
We are Invested in IIQ mainly because the company is developing an exosome platform technology that can be applied to three different markets:
- Research Tools (US$661M market size)
- Diagnostic Products (US$6.1BN market size)
- Therapeutics (US$55.3BN market size)
(Source)
Exosomes are tiny, microscopic extra-cellular “containers” released by ALL human cells.
Exosomes can carry ‘messages’ - clues as to whether or not someone has a disease - which is why IIQ has applied the platform tech to develop diagnostics products.
IIQ is also developing therapeutics to see if these "messengers" can be “weaponised” to locate and kill cancer cells.
IIQ’s first target in the therapeutics market is Triple Negative Breast Cancer (TNBC) - which currently has no approved cell therapies treating it, and where the current primary standard of care is chemotherapy.
What else is IIQ working on?
In the diagnostics space, we like IIQ’s ovarian cancer product the most.
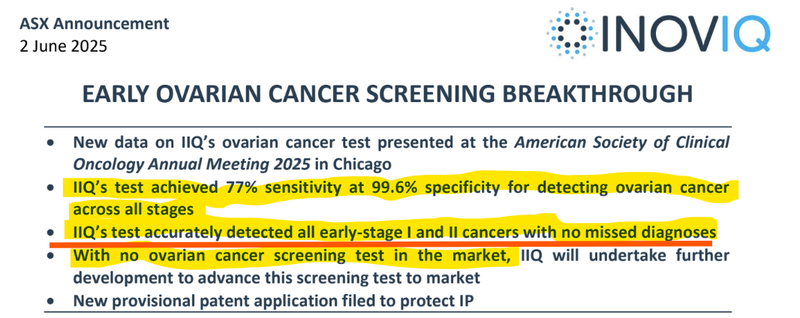
Earlier this year, IIQ released results that showed its ovarian cancer test was almost 100% accurate across 532 tests.
IIQ’s test accurately detected all early-stage I and II cancer with no missed diagnoses (the earliest stages).
(Source)
The next stage for IIQ is to partner with a clinical lab within the US as a central hub to undertake more tests and secure more data in anticipation of a clinical validation study - the final hurdle before FDA clearance.
IIQ is in discussions with leading clinical laboratories and diagnostic companies, and it is expected that EXO-OC will be Laboratory Developed Test (LDT) ready by the end of next year.
There is currently no recommended screening test for women who show no symptoms, so IIQ’s diagnostic (EXO-OC) has the potential to revolutionise ovarian cancer detection and drastically improve survival rates.
We did a deep dive on why we like IIQ’s Ovarian Cancer screening tool and how we think IIQ can commercialise the tool in our last IIQ note here.
What’s next for IIQ?
Here’s the rundown of future catalysts from a recent IIQ presentation:
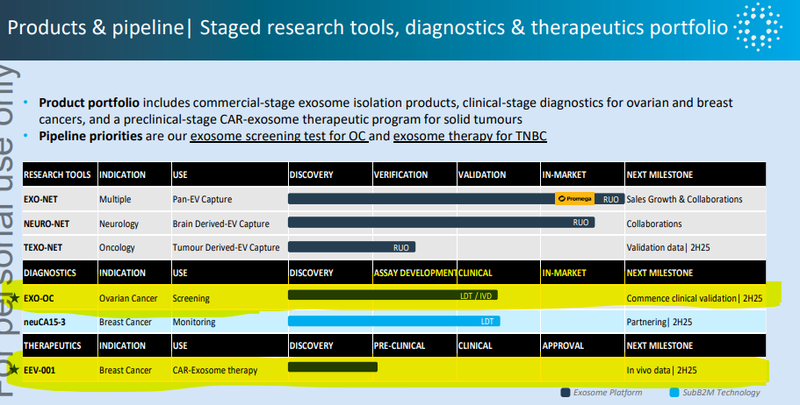
In particular, we are looking forward to the following catalysts:
- 🔄Updates on solid tumour therapy in vivo studies (results from today), IIQ confirmed today that there is more data on this expected to be released in 2026, with in human trials planned for 2028.
- 🔄A lab partner for SubB2M tests (for breast cancer monitoring)
- 🔄Clinical validation studies for highly accurate ovarian cancer screening test
- 🔄Development of exosome diagnostic for neurodegenerative conditions like Alzheimer’s and Parkinson's.