IIQ publishes data for its triple negative breast cancer therapeutic
Our biotech Investment Inoviq (ASX: IIQ) just announced that its data has been published in a peer reviewed journal
IIQ’s exosome-based platform technology is currently in development stages for cancer detection and cancer therapy.
Exosomes can carry ‘messages’ - clues as to whether or not someone has a disease - which is why IIQ has applied the platform tech to develop diagnostics products.
Today, IIQ published proof of concept data in the Journal of Visualised Experiments (JoVE), showing:
- The in vitro (in test tubes) cancer-killing efficacy of its engineered CAR-T-exosomes
- The scalability of its EXO-ACE manufacturing platform
Check out the full published journal here: Enhancing Chimeric Antigen Receptor-Extracellular Vesicles (CAR-EV) Technology: The Future of Cancer Therapy

(Source)
This comes only a few weeks after IIQ confirmed that its therapeutic was able to kill more than 90% of the cancer cells within 10 hours of treatment.
These results were from “in-vitro” (in test tube) studies.
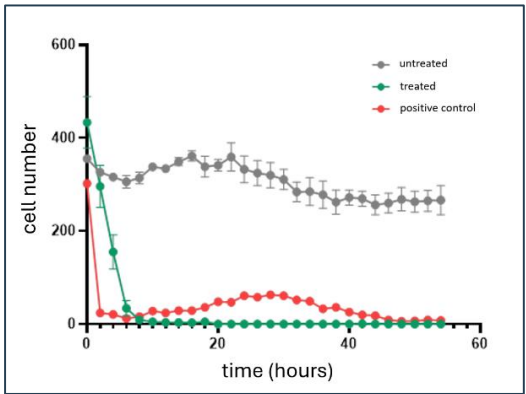
(Source)
Next, IIQ plans to test its therapeutic in animals - starting next quarter (Q4-2025).
Here is a slide we liked from a previous IIQ webinar which showed the development process for IIQ’s solid tumour therapeutic:
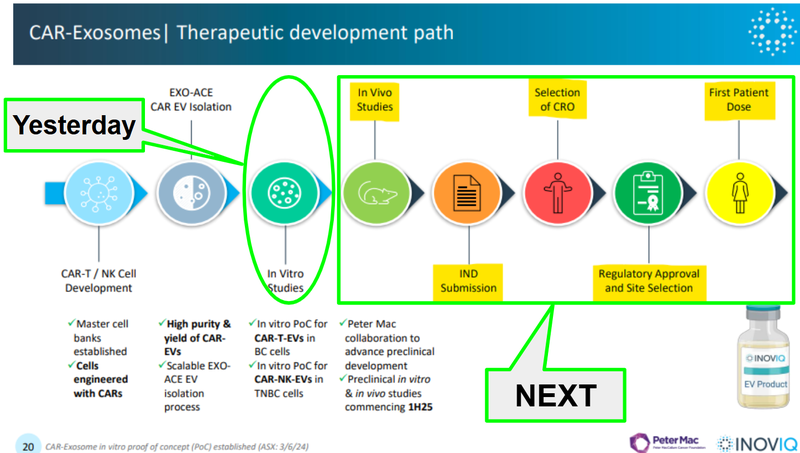
(Source)
What else is IIQ working on?
UniQuest worldwide licencing deal
Last week, IIQ exercised its option to secure an exclusive worldwide licence from UniQuest for the biomarkers used in this test. Read the full take here.
UniQuest is the commercialisation company of The University of Queensland, who turn research discoveries into commercial opportunities which has previously included cervical cancer vaccine Gardasil.
Details of the licencing terms include:
- Exclusive rights: Worldwide rights to develop and commercialise ovarian cancer exosome test IP.
- Licensed IP: Patent application (filed 29 May 2025 by UQ) covering novel protein/RNA biomarker combinations and methods, plus any improvements.
- Financial: $25k upfront, up to $360k in milestone payments, and tiered royalties up to 2.5% of net sales (excluding EXO-NET).
- Term: Longer of 10 years from first commercial sale or patent expiry.
This licence de-risks IIQ’s development and commercialisation pathway for the EXO-OC test.
The next stage for IIQ is to partner with a clinical lab within the US as a central hub to undertake more tests and secure more data in anticipation of a clinical validation study - the final hurdle before FDA clearance.
IIQ is in discussions with leading clinical laboratories and diagnostic companies, and it is expected that EXO-OC will be Laboratory Developed Test (LDT) ready by the end of next year.
There is currently no recommended screening test for women who show no symptoms, so IIQ’s diagnostic (EXO-OC) has the potential to revolutionise ovarian cancer detection and drastically improve survival rates.
What’s next for IIQ?
Here’s the rundown of future catalysts from a recent IIQ presentation:
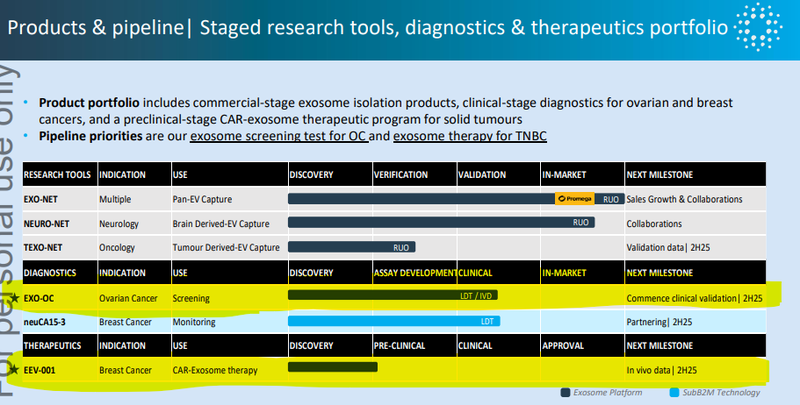
In particular we are looking forward to the following catalysts:
- 🔄Updates on solid tumour therapy in vivo studies (targeting Q4 of this year for in vivo results with Peter Mac)
- 🔄A lab partner for SubB2M tests (for breast cancer monitoring)
- 🔄Clinical validation studies for highly accurate ovarian cancer screening test
- 🔄Development of exosome diagnostic for neurodegenerative conditions like Alzheimer’s and Parkinson's.