IIQ establishes highly respected scientific advisory board
Today, our biotech Investment Inoviq (ASX: IIQ) announced the establishment of its scientific advisory board.
We see this as a strong sign that IIQ’s diagnostics and therapeutics platforms are maturing as they approach an important juncture where, ultimately, commercialisation becomes a more tangible prospect.
Here’s how we think IIQ’s new scientific advisory board can help it achieve its objectives:
- Strengthens INOVIQ's position in exosome technology, a key focus area for the company
- Adds credibility and expertise to development programs, potentially accelerating commercialization
- The guidance, contacts and industry knowledge the scientific advisory board offers could help IIQ achieve near-term catalysts expected in 2025, including validation data for various tests and potential partnerships
- Expertise in clinical trials and cancer treatment crucial for longer-term goals, such as clinical validation for ovarian cancer screening and first sales of breast cancer monitoring tests in 2025
IIQ’s new panel of experts have a strong mix of skills, across cancer therapeutics and diagnostics, exosomes and liquid biopsies.
IIQ’s product pipeline across all of these fields are at various stages of development, providing multiple “shots on goal” across a range of high Total Addressable Market (TAM) opportunities.
Among the newly appointed experts in IIQ’s are:
- Professor H. Miles Prince AM: Leading Clinical Haematologist and Oncologist and Professor at both Melbourne and Monash universities. He is an NHMRC Investigator Fellow and has been principal investigator of over 100 clinical trials including targeted therapeutics (CAR-T therapy) for haematological conditions and cancers.
- Professor Phillip K. Darcy: Group Leader of the Cancer Immunotherapy Laboratory at the Peter MacCallum Cancer Centre and NHMRC Principal Research Fellow, focusing on novel T cell-based immunotherapy approaches for cancer in preclinical mouse models and clinical translation.
- Professor Carlos Salomon: Director of the University of Queensland Centre for Extracellular Vesicle Nanomedicine, Head of the Translational Extracellular Vesicles in Obstetrics and Gynaecology Group and NHMRC Investigator Fellow, specialising in exosome biology and its clinical translation to diagnostics and therapeutics for ovarian cancer and obstetrical syndromes.
- Dr James McCracken: Leading Medical Oncologist specialising in breast cancer treatment at Epworth Healthcare and the Peter MacCallum Cancer Centre. His research interests include the field of liquid biopsies for cancer to personalise treatment and minimise toxicity.
How does this impact our IIQ Investment Memo?
We primarily see IIQ’s new scientific advisory board as strengthening one of the key reasons we Invested in IIQ, which we listed in our IIQ Investment Memo:
Large commercial opportunities from underlying technology
IIQ has an exosome isolation product which is already being sold to research groups. IIQ also has commercial agreements with Promega and a European biotech to bring IIQ’s exosome platform technology to market. Because IIQ’s exosome technologies can be applied to diagnosing and potentially treating many different diseases, we expect to see more partnerships where IIQ licences their exosome tech.
Source: 12 June 2024 IIQ Investment Memo
What’s next for IIQ?
We’ve highlighted a few of IIQ’s upcoming milestones this year that we are most interested in:
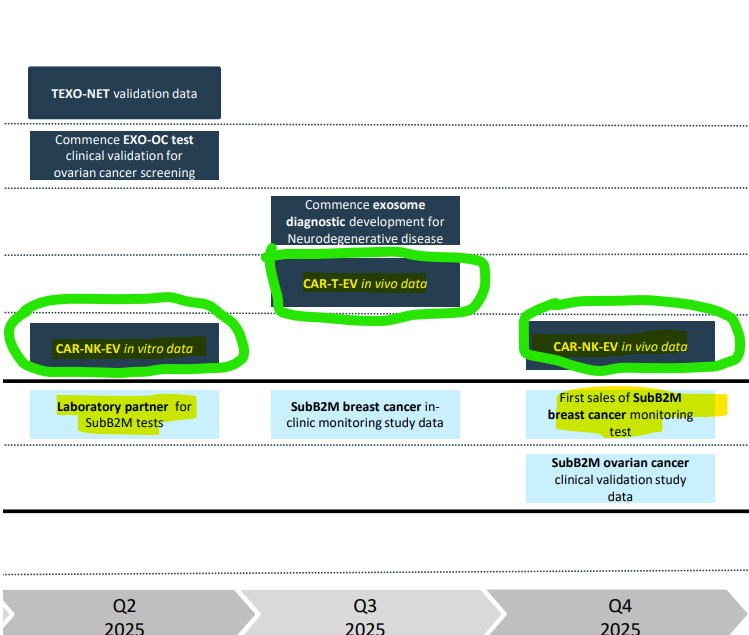
(Source)
In addition to progress across NEURO-NET and ovarian cancer, we are looking for two broad classes of updates:
- Therapeutics - we want to see IIQ’s cancer “moonshot” therapeutic which has been shown to kill solid tumour cancer cells in a test tube, transition to animal studies.
- Diagnostics - we want to see IIQ’s superior breast cancer test (SubB2M tests) pick up a lab partner to speed its path to market and first sales.
To get the SubB2M tests commercialised, the next steps “include publication of a scientific paper, transfer to a high-throughput instrument platform, additional in-clinic breast cancer monitoring study and securing a laboratory partner for commercialisation.”