NTI’s trial of Autism treatment in children shows further patient improvement after 12 weeks.
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 8,020,000 NTI shares and 350,000 NTI Options at the time of publishing this article. The Company has been engaged by NTI to share our commentary on the progress of our Investment in NTI over time.
Autism affects approximately 1 in 100 children around the world.
Negative symptoms include social difficulties, communication challenges, and repetitive/restricted behaviours for the child.
(which in addition create difficulties for parents, caregivers and family members)
There are currently no approved treatments available to help children with Autism Spectrum Disorder (ASD).
Our biotech Investment Neurotech International (ASX:NTI) is looking to change this.
NTI is taking its product - an IP protected, high potency cannabinoid formulation in oil - through a series of clinical trials to test its safety and efficacy for treating ASD.
(and a number of other neurological disorders in children in parallel)
Evidence of effectiveness in clinical trials is how companies like NTI can get regulatory approval for its treatments by the FDA in the USA and the TGA in Australia.
The $70M capped NTI is following a similar pathway to one of the most successful ASX biotechs in recent years, the $2.4BN capped Neuren - which has gone from $1 to $20/share.
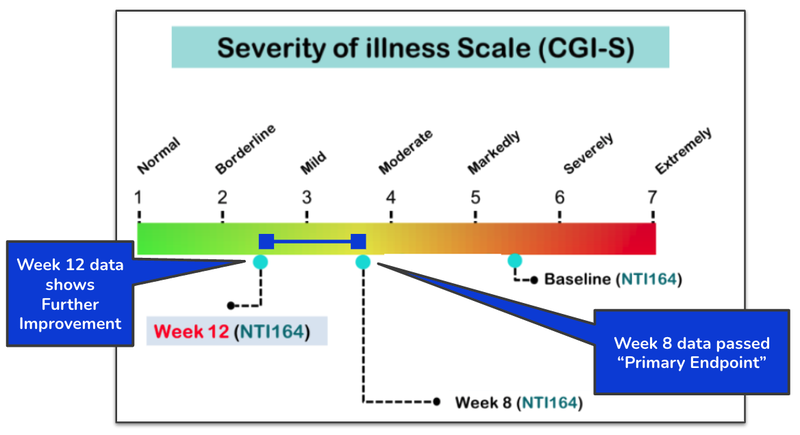
Yesterday, NTI published 12 week data from its Autism Spectrum Disorder treatment Phase II/III trial which shows meaningful reductions in the severity of the illness as time goes on.
After 12 weeks receiving NTI’s product, patients improved to the point where “ASD symptoms are present but barely noticeable”.
This is a significant improvement to the baseline observations of the children as “markedly ill”.
This new 12 week data is a significant upside to the already incredibly promising 8 week results on NTI’s ASD treatment:
In addition, patients who were on the placebo for the first period of the trial were switched to NTI’s treatment at the 8 week mark.
And improvement was shown immediately when the placebo group switch to NTI’s treatment:
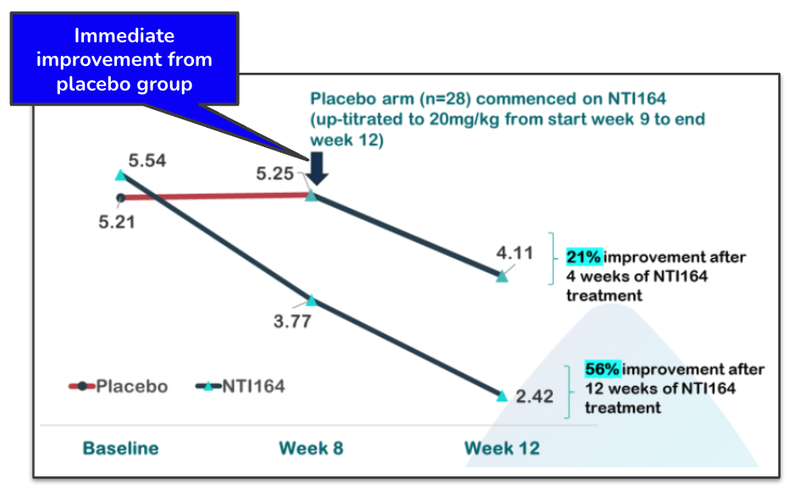
What this data shows is that NTI’s treatment continues to be effective over time at treating ASD, beyond what was considered statistically significant.
We already knew the initial 8 week results published by NTI in April were promising - the latest results are excellent in our opinion.
We were actually surprised the NTI share price didn’t respond to the 8 week news back in April.
(We suspect there might have been some long term holders selling into any liquidity - hopefully they are now finished and the NTI share price can re-rate off the next batch of newsflow).
There are currently no approved treatments available to people with ASD, so if NTI can prove its product is effective, it could prove to be valuable.
The next step for NTI in this trial is to collect the 18 week data, then 54 week data.
The major catalyst for NTI for this treatment will be to secure regulatory approvals so their ASD treatment can be sold to consumers.
In a nutshell, we think the extended results from the Phase II/III autism trial are highly encouraging and further validate NTI’s treatment as a potential breakthrough treatment for Autism Spectrum Disorder (ASD).
If NTI’s ASD treatment successfully makes it to market - we believe it could be life-changing for ASD patients and their families.
With 1 in 100 children affected by ASD, a safe and effective treatment like NTI164 could fill a major unmet need.
With no serious adverse events, and no adverse events attributable to NTI’s treatment, whilst there is more work to do, we think things are looking good for NTI.
In addition to ASD, as we hinted at above, NTI is working to treat a number of rare neurological disorders in children - we think catalysts in the next 6 months will be related to getting ‘orphan drug designation’ for multiple disorders with the regulators in the USA and Europe.
Today we will cover off all the latest on NTI, including a look at how NTI is progressing against the Investment Memo we wrote when we first Invested.
Autism Spectrum Disorder (ASD) is a developmental disorder that affects social interaction, communication and behaviour. Symptoms appear in early childhood and range in intensity.
- Causes: The exact cause of ASD is unknown, but generally believed to be a combination of genetic and environmental factors.
- Symptoms: Social difficulties, communication challenges, repetitive/restricted behaviours.
- Prevalence: ASD is a common disorder, affecting approximately 1 in 100 children around the world. The prevalence of Autism has been rising in recent times, but the cause is unclear.
- Treatments: There is no current cure for ASD, but early-stage intervention therapy like Applied Behavior Analysis (ABA) and speech therapy can improve outcomes. Medications can also be used for associated symptoms such as anxiety or aggression.
In April this year NTI announced that its Phase II/III autism trial had met the primary endpoint at the 8 week mark.
This was a big milestone for the company.
However, this new data, published, demonstrates that from weeks 8 to 12, the treatment's efficacy goes beyond what was needed to meet the primary endpoint.
The trial is designed for the patients to continue taking the treatment for up to 18 weeks and then undergo a " wash-through” period to evaluate for any safety issues.
From there, there will be an extension period that ends at the 54-week mark, where NTI will gather more data about its treatment over a longer period.
These results are very positive and show the potential for NTI’s drug to treat patients with Autism Spectrum Disorder.
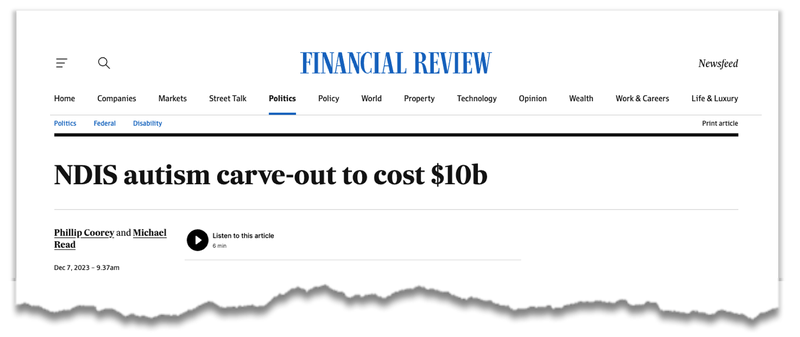
Autism and its cost to the NDIS (Australia’s National Disability Insurance Scheme) has been in the spotlight over the last 12 months.
Patients with autism account for nearly 35% of users of the $6BN NDIS (way more than ever envisioned).
The Australian government has set up a new $10BN scheme to treat children with ASD, and work is being done to find treatment solutions:
( Source )
The NDIS has been a big balance sheet blowout for the Australian government, and we think that NTI’s autism treatment could be a possible solution.
This will be the case that NTI presents to regulators for approval of its drug, together with the data.
Regulatory approval will mean NTI’s ASD treatment can be sold in the market and would be a major upcoming catalyst for NTI for this treatment.
However as we mentioned above, we think NTI news on orphan drug designation for Rett Syndrome, and PANDAS/PANS will be the nearer term catalysts for this stock in the coming months.
NTI developing treatments for multiple disorders
It has been a busy nine months for NTI.
NTI is progressing through the regulatory process for multiple neurological disorders in children - all with share price catalysts of their own.
To fund its progress, in April NTI raised $10M at 10c (we participated in the raise)...
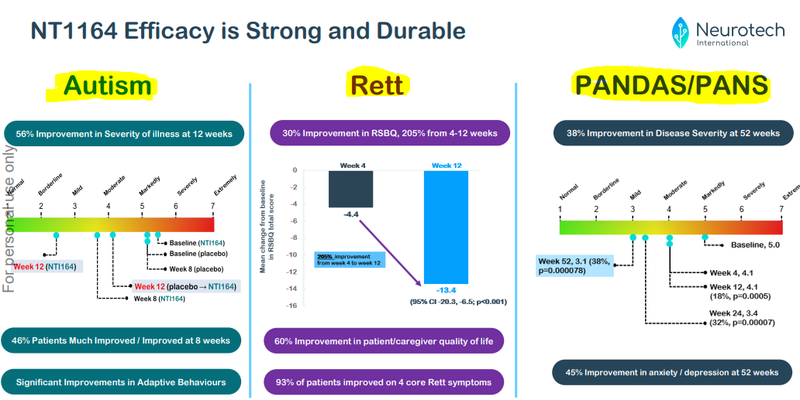
In recent months, NTI has published results from THREE clinical trials across three different rare (and severe) neurological disorders predominantly in children...
And all of NTI’s trials have met their primary endpoints.
( Source )
And the company continues to publish further results from the trials as they become available.
NTI is copying the playbook of ASX biotech darling Neuren Pharmaceuticals (capped at $2.5BN) which developed and commercialised a treatment for a rare disease in children by developing its own treatments for rare neurological diseases in children.
( Source )
Update on PANDAS/PANS -
In October last year, NTI published data showing that its treatment for PANDAS/PANS met the primary endpoint for both safety and efficacy.
These were very good initial results.
Since then, more data has been collected and further clinical benefits have been shown at the 52-week mark of the trial.
Both PANDAS and PANS are rare paediatric conditions.
- Causes: PANDAS is thought to be triggered by strep infections in children. Whereas, PANS can be set off by various infections, toxins, and other triggers. Both disorders are autoimmune in nature, where the child’s own cells mistakenly attack healthy brain cells.
- Symptoms: Sudden onset of OCD, tics, anxiety, emotional lability and some motor abnormalities. Symptoms may worsen acutely.
- Prevalence: Research suggests that PANDAS impacts 1 in every 200 children, however prevalence of PANS is less clear.
- Treatments: There is no simple treatment for PANDAS/PANS. Treatment usually involves antibiotics, anti-inflammatories and immunotherapy.
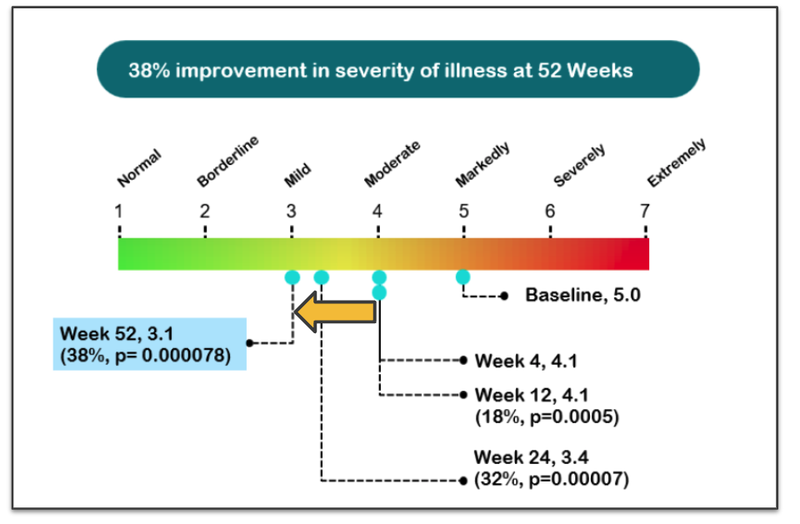
Like with the ASD trial, NTI met the primary endpoint but continued to gather data from patients over a longer period of time.
At the 52-week mark, there was a 38% improvement in severity of illness.
As time went on, the severity of the illness reduced.
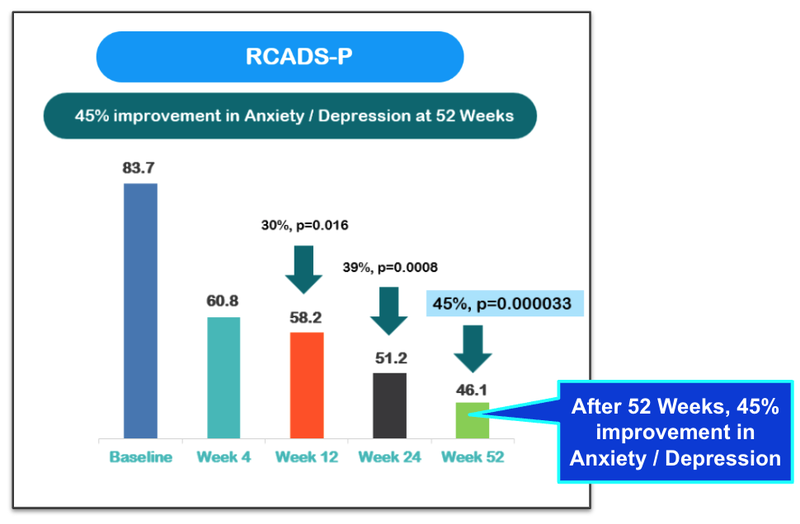
Also, there was a 45% improvement ins anxiety/depression across the cohort:
NTI included some quotes from caregivers of PANDAS/PANS children which provided a glowing endorsement of the impact that NTI’s treatment has had on their children.

( Source )
Since the results of the trial NTI has established an expert advisory group to provide guidance on its PANDAS/PANS drug development program.
We see this is a positive development, as the TGA has indicated a potential provisional registration pathway for NTI's lead drug candidate NTI164, which could accelerate approval by up to 2 years.
Ultimately, we want to see PANDAS/PANS be granted “Orphan Drug Status” in both Australia and the US.
This is important as it would provide a line of sight on the commercialisation of NTI’s treatment.
The annual market for PANDAS/PANS is worth US$1.2BN.
Providing access to treatment in Australia via TGA provisional registration would be a major milestone for NTI and an excellent outcome for PANDAS/PANS patients.
An entry into the Australian market would provide important bona fides to potential institutional investors, and we hope, a precursor to a breakthrough into more lucrative markets such as the US.
Results from NTI’s Rett Syndrome clinical trial
NTI’s third clinical trial results that it has published in the last nine months are for Rett Syndrome.
Rett Syndrome is a genetic neurological disorder which primarily impacts females between the ages of 6-18 months, and causes severe cognitive and physical impairments.
- Causes: Rett Syndrome is the result of mutations in the MECP2 gene (located in the X chromosome - hence more prevalent in females). The gene mutation is often sporadic, meaning it is not usually inherited from parents.
- Symptoms: Initial symptoms usually include loss of purposeful hand skills, reduced social engagement and slow growth of limbs. As the syndrome progresses, symptoms can include seizures, repetitive hand movements and intellectual disability.
- Prevalence: Rett syndrome is rare, usually impacting 1 in 10,000 live female births.
- Treatments: There is no cure for Rett Syndrome, but the core focus is to manage symptoms. Symptom management can include medications for anxiety, physical therapy and sometimes devices like limb braces. The recent drug pioneered by Neuren (NEU) targets treatment of Rett Syndrome.
Those of you who are keen investors in the biotech space might be familiar with “Rett Syndrome” - as it was the condition that put Neuren Pharmaceuticals on the map.
Neuren is the biggest biotech success story on the ASX in the past three years - NTI is following it
Neuren has grown over ~1,300% since it developed and commercialised its treatment for Rett Syndrome (a rare and nasty disease).
Neuren now has a market cap of $2.5BN and has offered the market a pathway to drug development success through the treatment of orphan diseases.
(below is an image we created a few months ago... Neuren is now actually near $20, not $15... )
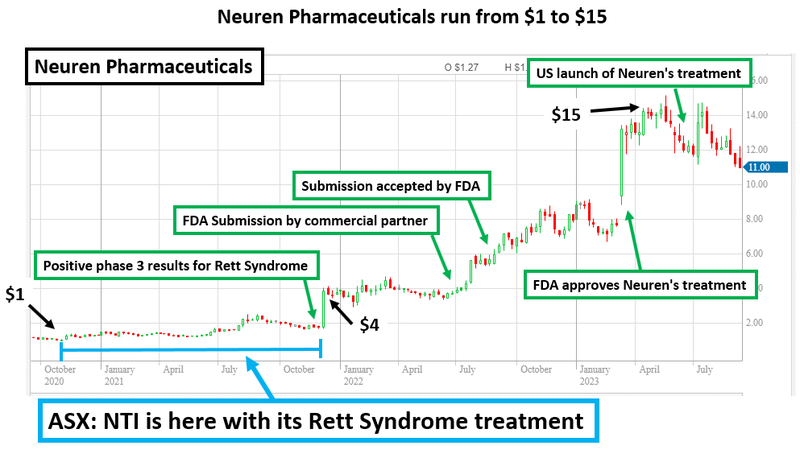
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
NTI is looking to become a “fast follower” of Neuren and address some of the safety concerns with its treatment.
Ultimately NTI’s results met the primary endpoint and were much safer when compared to Neuren’s product.
However, from an efficacy perspective, Neuren’s solution was superior.
For our full take on the results read: NTI’s Phase I/II Rett Syndrome clinical trial results improve upon $2.4BN capped Neuren’s results...
NTI appoints Chief Medical Officer
NTI also recently appointed a new Chief Medical Officer Carolyn Ellaway.
Carolyn Ellaway is a paediatrician and clinical geneticist with expertise in rare genetic disorders like Rett syndrome - the types of disorders NTI is looking to develop treatments for.
She was the lead investigator on NTI's successful Phase I/II Rett syndrome trial (essentially the lead scientist for the program).
In her role as CMO, Carolyn will be engaging with key opinion leaders and guiding NTI's clinical programs and regulatory strategies.
Carolyn brings with her experience in clinical genetics and paediatric experience.
Her area of expertise is the diagnosis and management of children with a wide range of rare genetic disorders including mitochondrial and lysosomal storage disorders, Rett syndrome and related disorders.
We think Carolyn’s set of expertise fits in perfectly with the paediatric focused treatments NTI is developing.
What is next for NTI?
As we said in our last note, NTI has always been good at signalling clearly what newsflow is to come, and delivering that newsflow on time.
Autism Spectrum Disorder
Week 18 and Week 52 data
NTI will publish further data at week 18 and week 52. We hope that this data improves on what we already know, that NTI’s treatment for ASD is safe and effective.
PANDAS/PANS
Metabologenomic data from Phase I/II PANDAS/PANS Clinical Trial
This is actually quite important - this is another measure of how well NTI’s treatment works for another rare paediatric disorder called PANDAS/PANS.
Results have already come back positive for this disorder in October of last year - this could show the method of action of NTI’s treatment, further strengthening its case for entry to market.
Orphan Drug Designation
NTI is seeking orphan drug designation for PANDAS/PANS.
To encourage the development of treatments of rare diseases, governments provide generous incentives to companies.
- Fast Tracked Approvals: This means that the company will not always need to undertake the entirety of a Phase III clinical trial in order to get the product to market.
- Market Exclusivity: Once the US FDA grants orphan drug designation to a drug, it also gets seven years of market exclusivity.
- Orphan drug pricing: Orphan drugs are priced at a point that encourages drug developers to make treatments.
We are hoping that NTI can secure orphan drug designation from PANDAS/PANS in Europe and the US.
Cerebral Palsy
Commence Phase I/II trial
We expect NTI to start recruiting for this trial which could add another big string to NTI’s bow.
NTI already has Human Research Ethics Committee (HREC) approval and Clinical Trial Notification (CTN) scheme clearance by the Therapeutic Goods Administration (TGA) for this trial.
Cerebral Palsy is a group of permanent movement neurological disorders, appearing in early childhood.
It impacts muscle coordination and motor-neuron skills, but can often impact other body parts too. No two people experience CP in the same way.
Rett Syndrome
FDA IND/EMA toxicology
US FDA Investigational New Drug and European Medicines Agency toxicology reports will hopefully show that NTI’s treatment is safe in the human body - this can only strengthen NTI’s case for market entry.
How does yesterday’s NTI news impact our Investment Memo?
Promising efficacy data at treating Autism, large addressable market
If NTI’s treatment is proven safe and effective for Autism Spectrum Disorder (ASD), we think it could become an important part of the overall care for ASD sufferers, which is 1 in 100 children.
Source: "Why did we Invest" Section - NTI Investment Memo 18 September 2023
We think NTI’s ASD treatment results will only strengthen the company’s case to regulators, potentially opening a very large addressable market, to complement the company’s more niche indications such as Rett Syndrome and PANDAS/PANS.
What could go wrong?
With NTI’s two clinical trials meeting their primary endpoints, and a $10M raise tucked away we now see two different risks, which are - “Regulatory risk” and “Market risk”.
What could go wrong - Market risk
Broader market sentiment for small pre-revenue biotechs could get worse and the sector as a whole trades lower, taking NTI’s share price with it. Alternatively, the entire market could sell down as well.
Source: “What could go wrong” section - NTI Investment Memo 18 September 2023
Regulatory risk:
We see the upcoming discussions with EU and US regulators about Orphan Drug Designation for PANDAS/PANS as key catalysts - regulators could decide that NTI’s drug does not qualify.
This one is outside of our NTI Investment Memo, but we think it is a key risk for the company over the next 6-12 months.
There is always a risk that the discussions with US/EU regulators will not go according to plan and that NTI’s drug will not be approved for Orphan Drug Designation.
If NTI doesn’t get these designations, the market could imply the treatments don't work and sell off NTI’s shares.
In this scenario we would expect the share price to re-rate lower.
Our NTI Investment Memo
Our Investment Memo provides a short, high-level summary of our reasons for Investing. We use this memo to track the progress of all our Investments over time.
In our NTI Investment Memo , you can find the following:
- What does NTI do?
- The macro theme for NTI
- Our NTI Big Bet
- What we want to see NTI achieve
- Why we are Invested in NTI
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

