News in the queue for IIQ - upcoming share price catalysts…
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 1,146,000 IIQ shares and 523,000 IIQ options at the time of publishing this article. The Company has been engaged by IIQ to share our commentary on the progress of our Investment in IIQ over time. Some shares and options may be subject to shareholder approval.
Our biotech Investments have been the shining lights in our Portfolio over the last 12 months.
Given this form, in June we took a pretty big swing at the INOVIQ (ASX:IIQ) placement and added IIQ to our biotech Portfolio.
(only our fifth new biotech addition in 3 years)
Our IIQ Entry Price is 50c and we hope to see the company deliver us a DXB (up ~500%) or ALA (up ~240%) style return.
(past performance is not an indicator of future performance)
IIQ is developing diagnostics AND treatments for cancer AND Alzheimer's disease.
(two out of the four most likely diseases to eventually kill a person)
These are enormous markets if IIQ can deliver successful diagnostics tools for early stage detection of the diseases (diagnosis)
...and then add treatments to cure the diseases (therapeutics).
We invested in IIQ following the lead of Merchant Capital Group, who has introduced us to our three most successful biotech Investments and are a 14.2% holder of IIQ.
With IIQ we also wanted to jump onto the coattails of the company’s chairman David Williams.
Back in February 2014 David joined a small ASX biotech called Polynovo and presided over a share price rise from ~10c to almost $4 over the following 6 years.
So we like that David Williams owns over 5% of IIQ and is also chairman.
We are hoping that David can deliver another Polynovo style outcome for shareholders with IIQ.
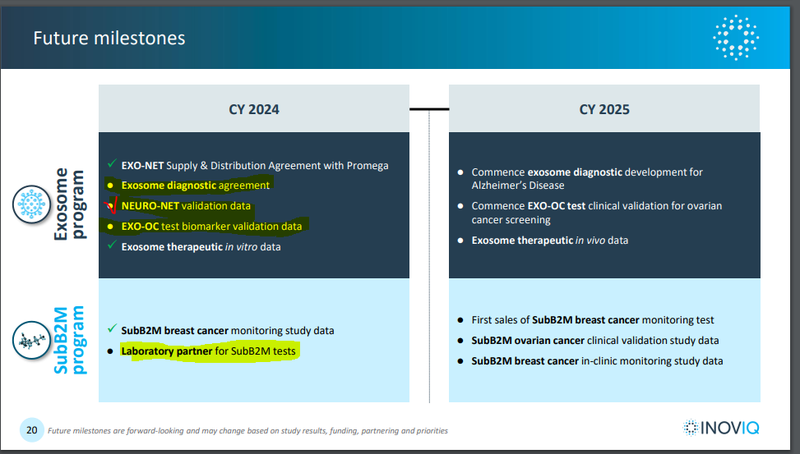
Upcoming IIQ share price catalysts we are watching out for are:
- A laboratory partner for its “SubB2M” breast cancer blood test - this test detects 19% more cancers than the leading FDA approved test. A lab partner would speed IIQ’s path to market and the prospect of making money. IIQ also has an ovarian cancer SubB2M test.
- An exosome diagnostic agreement - some form of commercial arrangement for IIQ’s exosome based tests. This would show that an external party sees the potential in IIQ’s exosome based tests.
- Exosome ovarian cancer test validation data - which will tell us how accurately IIQ’s test detects this disease.
(Source - IIQ Presentation 12 June 2024)
This quarter, IIQ made material progress across its research tools, diagnostics and therapeutics, and today we will look into how it has advanced on each of these fronts, and how it is tracking against our Investment Memo.
IIQ is developing and commercialising an exosome technology platform for breast cancer, ovarian cancer and neurodegenerative disease (Alzheimer’s) diagnostics as well as a solid tumour (breast cancer) therapy.
(we’ll explain what “exosomes” are later in this article)
In IIQ’s quarterly report that was released yesterday, we got a good summary of progress made, and a preview of what to expect in the coming months:
- A healthy cash balance of $9.2M, with an additional ~$2.4M received post June 30th
- Progress across diagnostics and therapeutics for cancer and Alzheimer’s
- Revenue traction from its Research Tools workstream which brought in $261k in the quarter (and $660k for the full year - growing this will help reduce IIQ’s net cash burn)
We are just 6 weeks into our Investment in IIQ and today we are going to assess IIQ’s progress against what we wanted to see in our Investment Memo.
You can read the full Investment Memo here: IIQ Investment Memo
Here are the here are the 9 reasons we Invested in IIQ back in June:
The 9 key reasons we Invested in IIQ:
- Going after big markets with potential breakthrough technology - IIQ is developing a technology that will detect cancer and possibly neuro diseases like Alzheimer’s. These are big markets and its platform technology could capture the interest of Big Pharma if it is able to prove that it works in a clinical setting.
- Strong board - the team from success story Polynovo - IIQ has three directors who are all with or previously with Polynovo, one of the great ASX biotech success stories of the last decade. Polynovo re-rated from ~4c to ~$4 in the space of 4 years and it remains capped at ~$1.7BN at the time of this memo.
- Merchant Funds Management are the biggest shareholder - Merchant is IIQ’s biggest shareholder holding ~14% of the company. Merchant has had a lot of success investing in Biotechs including - Race Oncology which went up ~2,900% (low to high) and the IIQ’s board other company Polynovo, up ~10,025% at its highest point.
- Platform technology to deliver multiple “shots on goal” - IIQ is somewhat different to a lot of biotechs out there in that its exosome technology platform doesn’t limit it to a binary outcome on a single clinical trial, if one catalyst doesn’t work out, there are more to look forward to.
- Tight cap structure - With just ~106M shares on issue and Merchant holding ~14% and Chairman David Williams holding ~5% there aren’t many shares floating around. This generally means that with fewer shares in the market, it’s more likely to re-rate on major news.
- IIQ’s breast cancer test is more accurate than leading test - A recent clinical validation study of IIQ’s breast cancer test showed that it detected 19% more breast cancers than the leading test (CA15-3 test by $332BN Roche) with an impressive 81% sensitivity and 93% specificity across a range of breast cancers.
- Early stage moonshot to treat solid tumours - IIQ is also advancing a solid tumour therapy which has been shown to kill 75% of breast cancer cells in 72hrs in an in vitro (test tube) study. There is a huge unmet need for treatments in the solid tumour space where, so far, cell therapies haven’t been developed.
- Expansion of tests to include ovarian cancer and neurodegenerative diseases (Alzheimer’s) - IIQ is looking to apply its exosome platform technology to ovarian cancer, and more recently has been working on neurodegenerative diseases such as Alzheimer’s. A blood test for the early detection of Alzheimer’s would be a major scientific advancement.
- Large commercial opportunities from underlying technology - IIQ has an exosome isolation product which is already being sold to research groups. IIQ also has commercial agreements with Promega and a European biotech to bring IIQ’s exosome platform technology to market. Because IIQ’s exosome technologies can be applied to diagnosing and potentially treating many different diseases, we expect to see more partnerships where IIQ licences their exosome tech.
You can read more in our original launch note on IIQ below:

Our New Investment is Inoviq (ASX: IIQ)
More on what IIQ does...
IIQ is developing and commercialising an exosome technology platform for breast cancer, ovarian cancer and neurodegenerative disease (Alzheimer’s) diagnostics as well as a solid tumour (breast cancer) therapy.
We’ve all been affected by cancer in some way...
And we know accurate and early testing is very important.
Breast cancer is the second most common cancer and early and regular tests can save lots of lives...
The most common blood test for breast cancer is produced by big pharma company Roche (capped at US$230BN).
The test was first created and developed during the 1980s - that's 4 decades ago - so improving on this product is a big undertaking...
IIQ has shown that its blood test detects 19% more breast cancers than the current leading test approved by the FDA.
And that’s just the start of what IIQ has developed...
The medical community needs these tests to better treat patients, and significant amounts of capital are being allocated in the market to get these tests into the market.
Recent deal values over the last few years for companies developing blood tests that accurately identify cancer range from US$250M in upfront payments to upwards of US$450M.
We like Investing in biotechs because they have the potential for large re-rates, and also come with an added feel-good factor that science can do great things for patients.
IIQ - capital raise complete
Yesterday IIQ put out its quarterly report showing a $9.2M cash balance at the end of June quarter, with a further $2.379M coming in after the quarter ended.
That comes after the company raised $7M from institutions last quarter at 50c/share followed by a heavily oversubscribed SPP.
The SPP was almost three times oversubscribed - IIQ ended up scaling back the demand and taking ~$2.4M of the $7.3M in applications.
Huge demand in SPPs is a good sign there are buyers out there for a stock.
In IIQ’s case we think the SPP had been putting downward pressure on the company’s share price during June, which is expected during an SPP.
SPP’s generally move buying pressure from existing shareholders “off-market” as they top up in the SPP instead of buying on market.
Now with the SPP done and 6 weeks passed since the placement, we are hoping the brakes are off IIQ’s share price, ahead of its upcoming news releases.
We think we are already seeing this, with IIQ was trading at 58.5c at last close, above the 50c placement price, which is a good sign there is demand.
We have already seen IIQ’s share price move higher off no news... so we are hoping it can move even higher off the back of some material catalysts being delivered.
IIQ - we are backing Merchant and David Williams
We think IIQ has the right backing & team to develop its exosome technology.
Merchant Group is IIQ’s biggest shareholder - we have successfully followed Merchant into DXB, ALA and NTI in the biotech space, and are hoping the form can continue with IIQ.
Merchant was a big investor in Polynovo - which re-rated from ~4c to ~$4 in the space of 4 years - a outsized ASX biotech success story that is currently capped at $1.8BN.
Interestingly - three of the four current IIQ board members ALSO come from Polynovo...
One of the Polynovo team (current Chairman), is also IIQ Chairman David Williams and a ~5% shareholder in IIQ...
With his track record of success, we’re Invested hoping to see a similar Polynovo style trajectory on the charts unfold for IIQ.
Of course, no guarantees here.
Below we’ve highlighted the Polynovo presence on the IIQ board:

(Source)
Earlier this month IIQ’s CEO Leearne Hinch presented to investors giving us a good explainer of what IIQ do, and idea of what to look out for next. You can watch the full video here:

(Source)
Ultimately we want to see IIQ take its exosome technology, commercialise it in the $6.1BN diagnostics market and hopefully apply it successfully in the US$55BN therapeutics market.
This brings us to our Big Bet for IIQ:
Our Big Bet for IIQ
“IIQ re-rates to a +$500M market cap on commercialisation of its breast cancer test, its ovarian cancer test, neurodegenerative disease test and/or its solid tumour (breast cancer) therapy.”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our IIQ Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
What is IIQ’s exosome technology?
IIQ is developing an exosome platform technology with far reaching implications across a range of diseases.
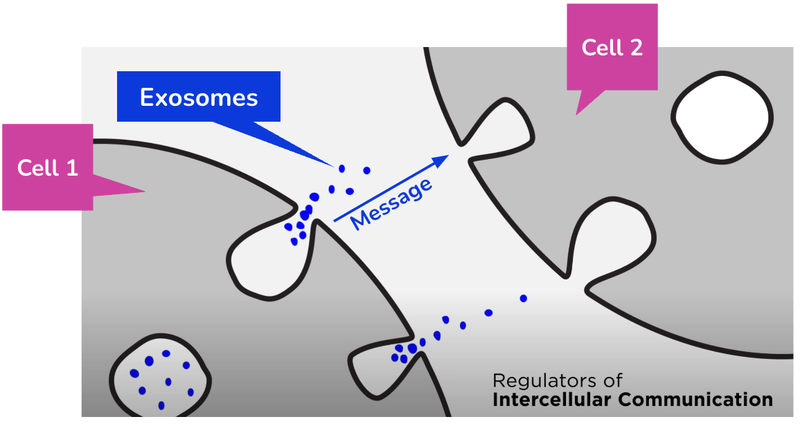
Exosomes are tiny parcels that carry messages between cells.
IIQ is developing technologies using exosomes to both diagnose (find what’s wrong) and treat (fix what’s wrong) various conditions.
Think of exosomes as the “couriers” or “postmen” to messages being delivered around the human body.
They pick up and drop off messages (biological information) to different parts of the body which triggers tailored responses from different cells...
IIQ uses these exosomes to develop various technologies that have the potential to diagnose and treat certain conditions.
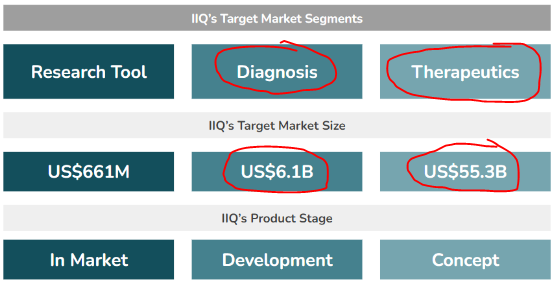
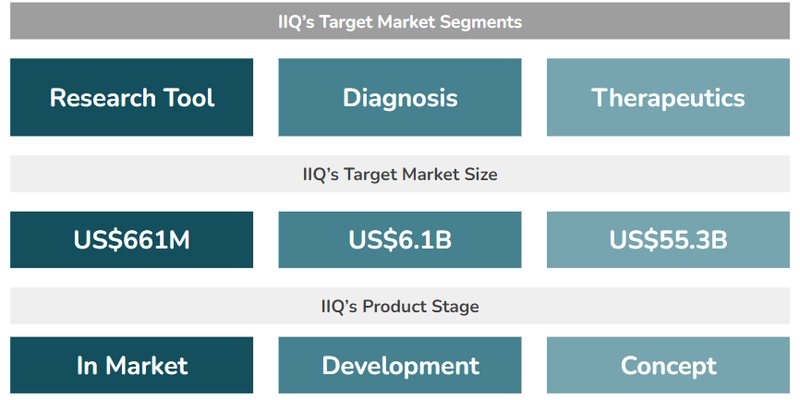
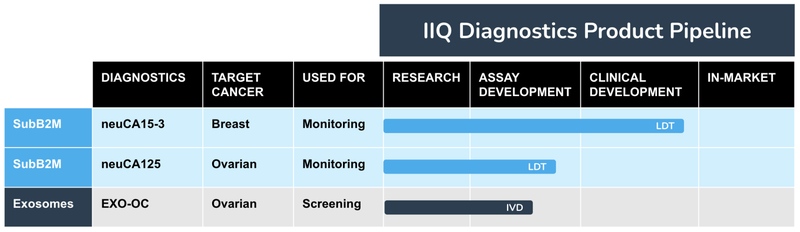
IIQ has three different business applications for its exosome technology:
- Research Tools(US$661M market size) - IIQ has an exosome-based tool used by researchers to enable biomarker discovery (identifying diseases) and validation (supporting the research of new treatments). This tool is already generating revenues for the company.
- Diagnostic Products (US$6.1BN market size) - IIQ is developing exosome-based tests for screening and monitoring for various conditions, including breast cancer, ovarian cancer and neurodegenerative diseases like Alzheimer’s.
- Therapeutics (US$55.3BN market size) - A “weaponised” exosome engineered to target and treat solid tumours. This is also in the development phase for IIQ.
As before, this is how we see these arms of IIQ’s three arms of its exosome product pipeline looking like:
This quarter, IIQ made material progress on each of these segments, and today we will look into how it has advanced on each of these fronts, and how it is tracking against our Investment Memo.
Our IIQ Investment Memo
As we mentioned above, when we first Invested in IIQ last month, we published our Investment Memo on the company.
This memo provides a short, high-level summary of our reasons for Investing.
We use this memo to track the progress of all our Investments over time.
In our IIQ Investment Memo, you can find the following:
- What does IIQ do?
- The macro theme for IIQ
- Our IIQ Big Bet
- What we want to see IIQ achieve
- Why we are Invested in IIQ
- The key risks to our Investment Thesis
- Our Investment Plan
Let's check in on how IIQ has been progressing.
How did IIQ progress its diagnostics tools?
Screening and monitoring for cancers
IIQ is developing tests to screen, diagnose and monitor breast cancer and ovarian cancer.
Screening is a very important part of the cancer detection process.
The earlier that the cancer is detected, the earlier that the patient can get treatment and prevent the cancer from spreading.
For those that have undergone treatment, it is important to monitor whether the cancer returns and if further treatment is needed.
Monitoring generally occurs four times a year for patients via a blood test.
The market size for screening and monitoring for breast and ovarian cancers is around US$6.1BN and is led by a big pharma company called Roche (which is capped at US$200BN).
IIQ is currently developing two tests for Ovarian Cancer, one for screening for cancer (with exosomes) the other for monitoring cancer (without exosomes).
With regards to the screening product (EXO-OC), IIQ’s clinical partner, the University of Queensland, has secured 500 samples for testing.
The validation study is set to be complete by the end of this calendar year, with the results to follow soon after.
With regards to the monitoring product (neuCA125), IIQ is following up on its validation study that was published in April with further clinical development.
The key milestone is for the company to receive a fit-for-purpose product which is currently in production with a contracted manufacturer.
Basically this means it needs more of its product to run a clinical trial.
Referring back to our Investment Memo, IIQ progressed on two fronts for our Objective #2: Advance Ovarian Cancer Tests:
Objective #2: Advance ovarian cancer tests (diagnostics)
IIQ has two ovarian cancer tests it is working on “EXO-OC” which is part of the company’s exosome work stream and a more advanced test called neuCA125 which is part of the SubB2M workstream and more similar to the breast cancer test above. The SubB2M ovarian cancer test is closer to market.
Milestones
🔲 EXO-OC test biomarker validation data
🔲 ➡ 🔄 Commence EXO-OC test clinical validation for ovarian cancer screening
🔲 ➡ 🔄 Laboratory partner to bring to market SubB2M ovarian cancer test to market
🔲 SubB2M ovarian cancer clinical validation study data
What do we expect IIQ to deliver? IIQ Investment Memo 12-June-2024
IIQ is also developing a breast cancer monitoring tool
IIQ’s product has been clinically validated to detect breast cancer across all stages, key breast cancer types and sub-types.
IIQ said that it has “initiated discussions with potential partners and key opinion leaders to secure a laboratory partner for commercialisation of the test”.
This demonstrates that IIQ is making progress on our Objective #1 for IIQ: Commercialise Breast Cancer Test.
Objective #1: Commercialise breast cancer test (diagnostics)
We want to see IIQ commercialise its breast cancer test (which has been shown to be more accurate than the ~$332BN capped Roche’s test) through the following additional milestones.
Milestones
🔲 ➡ 🔄 Laboratory partner to bring to market SubB2M breast cancer test to market
🔲 Real world data to support clinical adoption
🔲 Partnership with major testing provider to commercialise at scale
What do we expect IIQ to deliver? IIQ Investment Memo 12-June-2024
Screening and monitoring for Alzheimer’s Disease
Alzheimer’s is widely regarded as one of the most pressing medical problems in the world as the world’s population ages.
And last month, IIQ announced that its exosome platform could be used to help diagnose Alzheimer’s.
The data was published on the same day that we added IIQ to our portfolio, so we didn’t have a chance to go deep into the results... but it looks extremely promising.

(Source)
IIQ has developed a product called NEURO-NET, that isolates brain-derived exosomes.
Because of their tiny size, exosomes are able to cross the “blood-brain barrier” and potentially, provide a fingerprint of the health or disease status of the brain.
Here are the key takeaways from the announcement:
- Exosomes isolated from blood using NEURO-NET technology contain proteins known to be expressed by brain cells;
- NEURO-NET was superior to other methods tested in isolating brain-derived exosomes from blood;
- NEURO-NET could identify known Alzheimer’s biomarkers in exosomes that could not be detected by other methods;
- Analysis of NEURO-NET-captured exosomes identified more than 200 proteins that were differentially expressed in Alzheimer’s patients when compared with healthy individuals.
Below is a “map” of proteins that IIQ Alzheimer’s product was able to isolate:
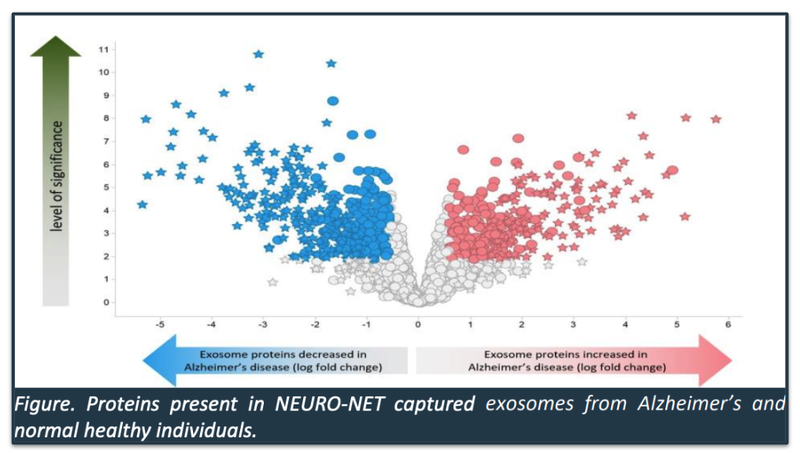
IIQ will now be putting AI to work on understanding this map, saying in the announcement that “these proteins will be combined using AI algorithms to develop a potential exosome-based blood test for Alzheimer’s Disease.”
Ultimately, we want to see this data form the basis for IIQ to develop an Alzheimer’s diagnostics tool.
(Noting that a cure for Alzheimer’s may be a long way off)
There are a range of drugs used to treat Alzheimer’s, but if doctor’s can’t pick up the signs early, their effectiveness is diminished.
And the medical community is certainly in strong need of a blood test for Alzheimer’s...
Alzheimer’s blood tests have received major media coverage this week:
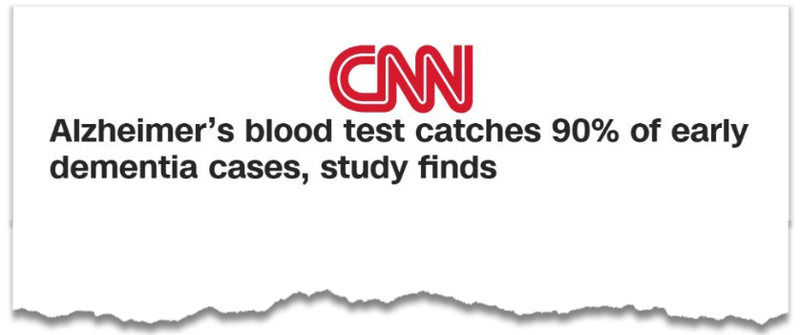
(Source)
The blood tests which garnered the media attention this week were focussed on a protein called “p tau-217”, and showed a “90% accuracy rate in determining whether memory loss is due to Alzheimer’s disease.”
Which is 17% more accurate than neurologists and other memory specialists.
Clearly, subjective human assessments of cognitive decline only go so far...
And in medicine, a better diagnosis means a better treatment - early detection is key here.
One study found that “By 2033, if a primary care doctor uses only the current cognitive assessments to determine dementia, people will wait an average of nearly six years before finding out whether they are eligible for new Alzheimer’s treatments.” (Source)
That kind of wait could have a major impact on patients’ lives.
So could IIQ’s exosome technology eventually unlock a commercialisable Alzheimer’s blood test?
Well, maybe.
That would be a huge outcome, and is the kind of thing that small cap investors like to see as the potential rewards would be large.
Of course at the same time it's no guarantee to occur - IIQ is a high risk investment.
IIQ’s progress this quarter on that front advances Objective #3: Advance neurodegenerative disease test (Alzheimer’s) in our IIQ Investment Memo.
Milestones
✅NEURO-NET validation data (Alzheimer’s test)
🔲Start exosome diagnostic development for Alzheimer’s Disease
Source: What do we expect IIQ to deliver? Section - IIQ Investment Memo 12-June-2024
How did IIQ progress its therapeutics tool?
IIQ is developing an exosome therapeutic to target and kill cancer.
IIQ’s solid tumour therapy has been shown to kill 75% of breast cancer cells in 72 hours in an in vitro (test tube) study.
Meaning that it hasn’t even been tested in mice... yet.
(Testing in mice in biotechnology is often the precursor to a human trial further down the track.)
There is a huge unmet need for treatments in the solid tumour space where, so far, cell therapies haven’t been successfully developed.
IIQ’s therapy shapes as a way to potentially cut out the nasty side effects of chemotherapy - which we think is the equivalent of burning down the house to kill a cockroach.
The most common form of cell therapy is CAR T therapy, which is good at targeting blood cancers but not so good at targeting solid tumours.
Solid tumours make up 90% of all cancers in adults and are the “final boss” for cell therapy platforms.
Where exosomes have an advantage over CAR T therapies is in their size.
Exosomes are tiny “sacs” that range between 30 to 150 nanometers in size.
Which is nearly 100 times smaller than a cell.
Because the exosomes are so small, the theory is that they will be able to breach into tumours and kill them.
IIQ published the results of its in-vivo proof of concept study at the start of June.
First, IIQ was able to show that its product isolated the exosomes from an immune cell with the cancer killing protein.
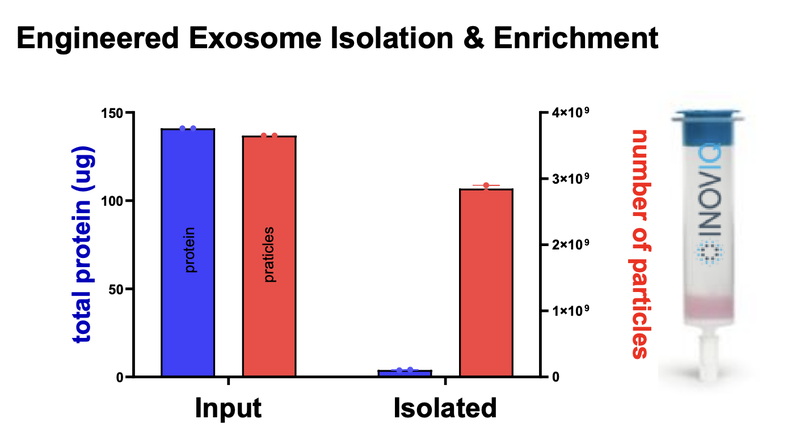
AND, that when the cancer was treated with these exosomes showed 75% cell death:
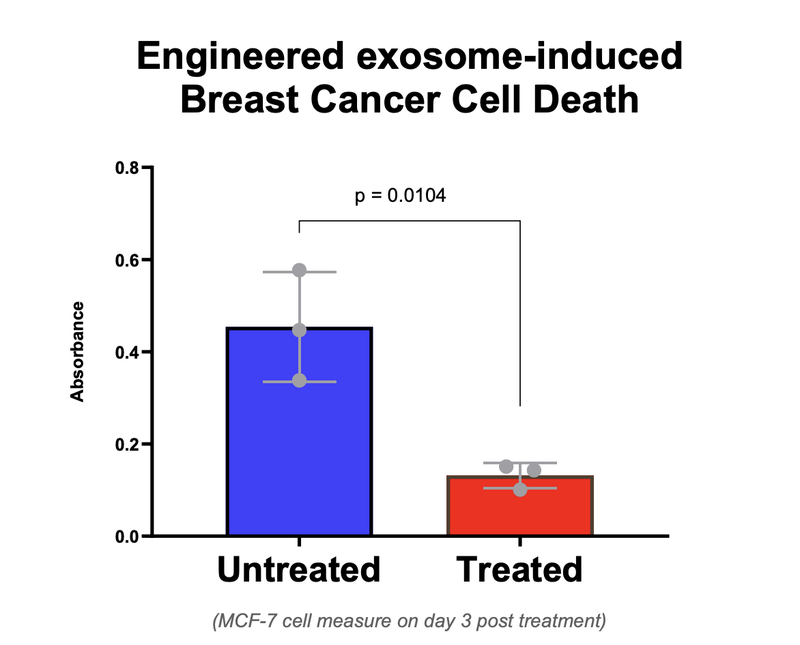
These are very promising results that we want to see the company follow up on as part of its preclinical research work on its solid tumour therapy.
Developing this technology is the “blue sky” scenario for IIQ and advances on Objective #4: Advance solid tumour (breast cancer) therapy from our IIQ Investment Memo
Milestones
🔲 Commence in vivo (mice) study
🔲 Report in vivo (mice) study results
Source: What do we expect IIQ to deliver? Section - IIQ Investment Memo 12-June-2024
How did IIQ progress its in-market product: Exosomes to support research
IIQ has a technology called EXO-NET which is used by researchers to help with drug development (think universities, clinical trial labs, R&D departments in big pharma).
The technology is a set of tools for isolating exosomes to identify and validate various biomarkers which are naturally occurring biological material that helps identify certain diseases.
IIQ has already received its first order from Promega, who is selling IIQ EXO-NET kits world-wide.
So far this technology has delivered $261,000 in revenue for IIQ this quarter, $660,000 for the full year. Although this isn’t game changing for the grander ambitions of IIQ, we are hopeful that this revenue will grow over time, especially if Promega’s sales really kick into gear.
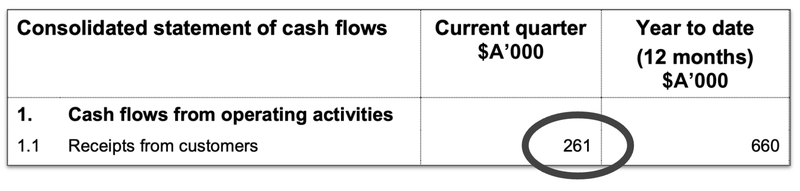
(Source - IIQ 4C Report, 29 Jul 2024)
Ultimately, we want to see IIQ sign some more large commercial deals for EXO-NET.
Objective #5: Large commercial agreement for Exo-NET (Research tools)
This is the product and associated services that are already in-market. IIQ has signed a marketing agreement with major biotech product manufacturer and distributor Promega as well as ResearchDx a US contract diagnostics organisation to get the research tools into the hands of customers. The exosome industry is growing quickly and we want IIQ to sign material agreements.
Milestones
🔲 Material agreement #1
🔲 Material agreement #2
Source: What do we expect IIQ to deliver? Section - IIQ Investment Memo 12-June-2024
What’s next for IIQ?
What we want to see next from IIQ is:
- A laboratory partner for its “SubB2M” breast cancer blood test which detects 19% more cancers than the leading FDA approved test. This would speed IIQ’s path to market and the prospect of making money. IIQ also has an ovarian cancer SubB2M test.
- An exosome diagnostic agreement - some form of commercial arrangement for IIQ’s exosome based tests. This would show that an external party sees the potential in IIQ’s exosome based tests.
- Exosome ovarian cancer test validation data - which will tell us how accurately IIQ’s test detects this disease.
Below is a timeline of these potential catalysts:

(Source)
While we know scientific research doesn’t proceed in a straight line, this year we are hoping IIQ can make progress in securing a laboratory partner for its “SubB2M” tests for breast cancer (and ovarian cancer).
A laboratory partner would help bring IIQ's test to market.
The recent deal sizes for liquid biopsies (blood tests) for cancer hover in the US$250-US$450M in upfront payment range:
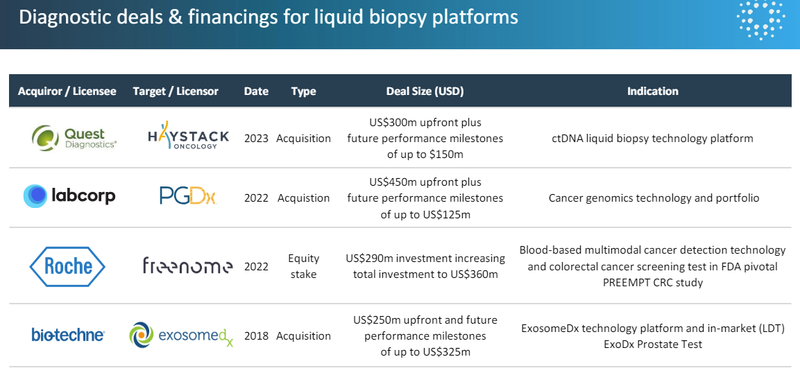
(Source)
And those deal sizes are all at multiples of IIQ’s current market cap.
While those deal sizes do look good - things can also go wrong...
What could go wrong?
Early stage biotech risk
This mainly applies to the solid tumour (breast cancer) therapy, which is currently in the preclinical phase of its development - noting that clinical validation for the diagnostic arm of IIQ’s business can still go wrong.
There are some standard risks that are associated with early stage biotechs, although the treatment appears effective in a test tube, this might not translate to humans. Key risks:
The treatment is ineffective
The treatment is not considered safe for human use
Patient recruitment is delayed
Ethics approval is delayed
Source: What could go wrong? Section - IIQ Investment Memo 12-June-2024
Additionally, another risk we are conscious of is “commercialisation risk” - meaning IIQ may find it difficult to bring its diagnostics into the market or other competitor products could crowd out IIQ’s product offerings.
We’re looking for near term traction on a laboratory partner for its SubB2M tests to reduce this risk.
Our IIQ Investment Memo
You can read our Investment Memo in the link below.
This memo provides a short, high-level summary of our reasons for Investing.We use this memo to track the progress of all our Investments over time.
In our IIQ Investment Memo, you can find the following:
- What does IIQ do?
- The macro theme for IIQ
- Our IIQ Big Bet
- What we want to see IIQ achieve
- Why we are Invested in IIQ
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

