Neurotech poised to commence clinical trials in March quarter
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
The last month has been an extremely rewarding period for Neurotech International Limited (ASX:NTI) with the company commencing stage 1 clinical product formulation and development studies in mid-November with a view to entering into clinical trials in the March quarter of 2021.
By the end of the month, management was able to report that further preliminary results of in-vitro human brain cell studies using its proprietary DOLCE/NTI leads indicated that they can regulate multiple neuronal pathways that are directly involved in cell repair and rejuvenation.
This followed successful preliminary results of in-vitro studies in human neuronal cell studies, positioning the clinical product development program to optimise and develop the most suitable product for phase 1 clinical trial program.
Commenting in mid-November on early stage developments and their impact in terms of shaping the future for Neurotech, as well as assisting the group in conducting a successful capital raising, chairman Brian Leedman said, “Our recently released interim results demonstrating the potent neuro-protective effects of our unique cannabis strains has enabled a successful capital raising for the planned commencement of Phase 1 clinical studies early next year.”
“The initiation of stage 1 product and formulation studies are vital steps before we can enter clinical studies and are designed to provide clinicians with the best options in respect to dosage and delivery system”.
Since then, Neurotech’s progress has been outstanding, and the company is shaping up as one of the most compelling emerging stocks in the biotech space.
The significance of the group’s achievements hasn’t been missed by investors with the company’s shares surging 130% to a high of 6.6 cents in the last fortnight, a level it hasn’t traded at for more than two years.
Two key emerging themes - medicinal cannabis and home therapy
While Neurotech’s progress in this high profile industry have substantial merit in their own right, it is essential to understand the driving forces behind the company’s success, as well as the group’s capacity to service high volume markets.
Essentially, Neurotech is a medicinal cannabis company, conducting clinical studies to assess the neuro-protective, anti-inflammatory and neuro-modulatory activities of its proprietary DOLCE/NTI cannabis strains which include CBDA, CBDP and CBDB.
The licensed strains contain < 0.3% THC, providing a clearer pathway to regulatory approval as compared to all other cannabis companies that contain far higher levels of THC.
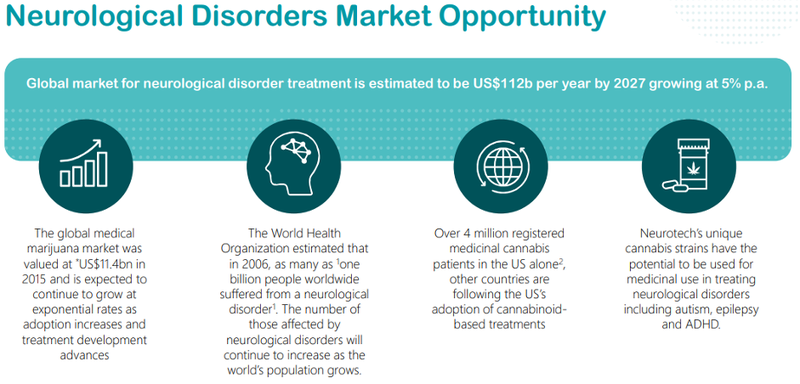
This comes at a time when there is increasing recognition by the medical fraternity of the benefits of medicinal cannabis in treating neurological conditions and assisting with mental health problems.
In tandem with this has been an increased acceptance by regulatory authorities of the benefits of CBD, such that previous restrictions that made the drug difficult to access have been lifted and/or eased in many global jurisdictions, including the US, Canada, the European Union and indeed Australia.
This has prompted analysts to revisit forecasts regarding the industry’s growth profile in coming years, but with significant developments occurring in the European Union and the US in recent weeks we can expect to see these numbers increase further.
However, as indicated below the global market for neurological disorder treatment was historically estimated to be US$112 million per year by 2027 based on a growth rate of 5% per annum.
Against this backdrop, the medical marijuana market was valued at US$11.4 billion in 2015, representing about 10% of the aforementioned estimated size of the neurological disorder treatment market in 2027.
The significant drain on government health systems, both in human resources and the cost of development and supply of devices for neurological disorders suggests that regulatory bodies will increasingly embrace new technologies that are safe, efficient and less taxing on public medical services.
Neurotech to benefit from home-based use devices
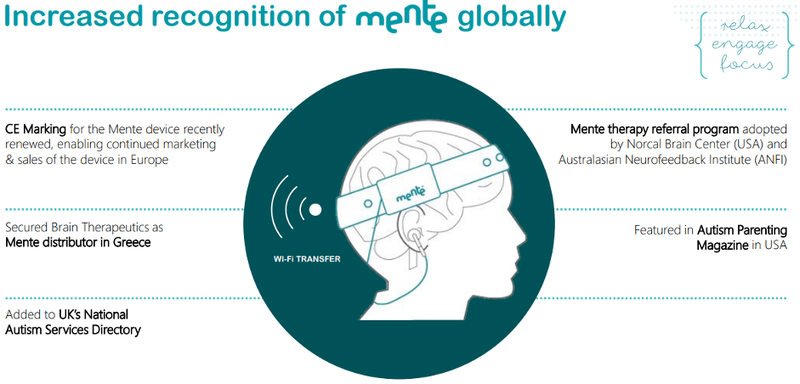
On this note, Neurotech is also commercialising Mente, the world’s first home therapy that is clinically proven to increase engagement and improve relaxation in autistic children with elevated Delta band brain activity.
The in-home aspect of the company’s approach is interesting in light of the success of numerous ASX-listed and global enterprises that have met the demand for home use services and devices due to mobility restraints resulting from COVID.
For example, shares in MyFiziq (ASX:MYQ) which provides personalised health and wellness technologies that can be used in the home has seen its shares surge more than ten-fold since March as the group formed collaborative agreements with other companies that were targeting similar markets.
Mente uses personalised audio feedback and earphones to gently guide the brain into a more relaxed state.
Based on autism research and a successful clinical trial, Mente is a soft, easy to use and portable headband that was developed to help those on the autism spectrum better self-regulate attention and mood.
Mente’s daily 40-minute sessions are personalised to the user’s current brain activity and have been designed to minimise any disruption to their daily routine, allowing them to continue with quiet activities (reading, drawing, school work etc) whilst still receiving therapeutic benefits.
This makes Mente perfect for use both at home and within an educational setting.
DOLCE/NTI leads more effective than use of CBD alone
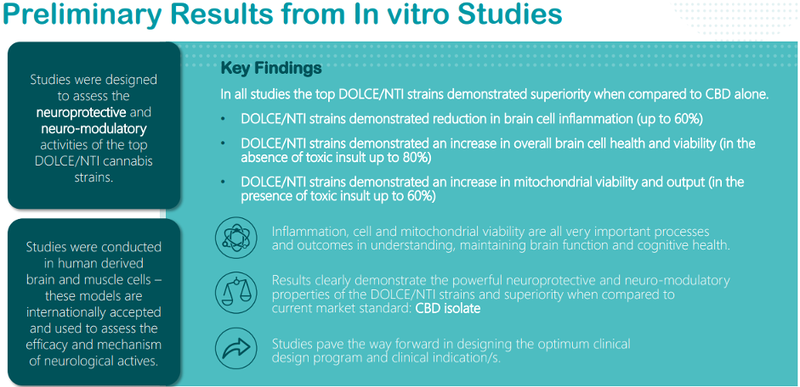
Harking back to Neurotech’s more recent success with preliminary in-vitro studies, subsequent results of in-vitro human brain cell studies using its proprietary DOLCE/NTI cannabis leads have proven very promising.
Importantly, the company has been collaborating with high profile medical research organisations in completing these studies.
Neurotech has been undertaking a series of in-vitro studies to assess the neuro-protective, anti-inflammatory and neuro-modulatory activities of the proprietary DOLCE/NTI cannabis leads which include, CBDA, CBDP and CBDB conducted at three leading independent laboratories – Monash University, University of Wollongong and RMIT University.
These studies provide further data to demonstrate the potential uniqueness of the DOLCE/NTI leads in their mode of action and their differentiation when compared to CBD alone.
Studies have indicated that the DOLCE/NTI leads regulate multiple neuronal pathways which are directly involved in cell repair and rejuvenation.
These leads indicate significant increased potency in repairing neuronal cells when compared to CBD alone.
More importantly, concentrations five times lower of the DOLCE/NTI leads are needed to achieve these results when compared to CBD alone (2ug/ml versus 10ug/ml respectively).
DOLCE/NTI leads work through a novel (different to CBD) mechanism, and they were shown to work through the powerful anti-inflammatory enzyme known as Arginase-1.
Conversely, CBD alone did not produce any significant anti-inflammatory properties.
DOLCE/NTI leads also increased the presence of beta-tubulin, an essential protein in the maintenance and healthy survival of brain cells.
In these studies, CBD alone did not produce an increase in beta-tubulin, a vital protein in the management of a number of chronic neurological disorders such as Alzheimer’s, Huntington’s and Multiple Sclerosis.
Final in-vitro results expected in coming weeks
Based on these findings, the studies provide further data to demonstrate the potential uniqueness of the DOLCE/NTI leads in their mode of action and their differentiation when compared to CBD alone.
This indicates that Neurotech is now positioned to fine tune its upcoming clinical program, selecting the optimum dose and patient parameters.
Most importantly, the DOLCE/NTI leads contain very low levels of THC (less than 0.3%) which may result in a less onerous regulatory process and therefore a quicker path to commercialisation.
Commenting on the highly promising results as well as pointing to the potential for applications in other areas, Leedman said, “These preliminary trial results continue to be very encouraging, in particular the unique mode of action of our strains compared to CBD alone.”
“These results indicate that the DOLCE/NTI leads may have a broader application in relation to the management and treatment of a number of neurological disorders”.
With the final results from in-vitro trials imminent there is the potential for further share price momentum, even though the stock has fired up over the last month.
While preliminary tests have the potential to provide share price reratings, the transition to clinical trials is a major milestone in its own right, as well as being the source of positive newsflow throughout the trial process.
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

