Imugene Meets All Endpoints in Phase 1b Gastric Cancer Immuno-oncology Trial
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
There is some fantastic work being done by medical researchers in a whole range of cancer-related fields that is taking us several steps closer to relieving or reducing symptoms.
The importance of this shouldn’t be underestimated. While scientists are some way from discovering the ultimate panacea — the cure for cancer — a reduction in symptoms has its own benefits.
Just think about the statistics.
According to the Cancer Council of Australia, an estimated 138,000 new cases of cancer will be diagnosed in Australia this year, with that number set to rise to 150,000 by 2020. The cost burden of this is more than $4.5 billion in direct health system costs (6.9%).
So any positive move in this area is welcome and would no doubt help patients, but also alleviate economic pressures.
Imugene (ASX:IMU), a clinical stage immuno-oncology company, is one such company that is working hard to reduce the impact of cancer symptoms, particularly gastric cancer. Today, the company delivered some exceptional news.
IMU has now released results from the Phase 1b Gastric Cancer trial, showing that all dose levels of its HER-Vaxx cancer vaccine showed an increase in antibodies in patients.
The preliminary topline results are even more impressive...
Of the 10 patients evaluable for tumour growth assessment during the study, 4 showed stable disease (SD), and a further 5 showed partial response (PR) for their best overall response.
A partial tumour response means a reduction in the total tumour burden by greater than 30%. A stable disease response means the total tumour burden either increases less than 20% or decreases less than 30%.
Note that this means an impressive 9 out of the 10 patients responded to the treatment — which is excellent going for a Phase 1b clinical asset like HER-Vaxx.
With the full results now in for the Phase 1b trial, IMU has entered advanced preparations stage for the Phase 2 study to start early in 2019.
Phase 1b met all endpoints for the study: checking for safety, tolerability, as well as identifying the optimal dose for the HER-Vaxx vaccine for Phase 2.
To refresh your memory: the 14 patient study tested three dose levels of HER-Vaxx (10, 30 and 50 micrograms) in combination with current standard of care chemotherapy, Cisplatin and Fluorouracil or Capecitabine.
Ten patients were evaluable for tumour growth assessment during the study – and, importantly, researchers saw an increase of antibody levels (immune response) in all patients, at all dose levels.
As the company continues to reach milestone after milestone, it seems there’s a significant clutch of shareholders keeping a close eye on IMU.
Following two key announcements in mid-November — IMU’s A$1.85 million R&D tax rebate, and the early positive results of Phase 1b trial — the small cap’s share price climbed a total of 20% over 10 days (peaking at $0.024).
In fact, its share price has climbed ~47% year-to-date.
Immunotherapy has emerged as one of the standard treatment modalities to fight cancer alongside surgery, radiotherapy, chemotherapy and other targeted therapies. Essentially, it’s a field where some of the smartest people in the world are figuring out how to allow the human immune system to better attack cancer when it invades.
Several of these people are working directly with IMU. They include four recent high-profile appointments: Professor Tanios Bekaii-Saab of the Mayo Clinic College of Medicine and Science; and world-renowned oncologist and researcher Dr Josep Tabernero, who just happens to be President of the illustrious European Society for Medical Oncology (ESMO).
IMU also has an impressive connection to one of the 2018 Nobel Prize Winners, James P. Allison, through its non-executive director, Axel Hoos.
That’s a strong stable and when you consider the fact that the market is hungry for cancer immunotherapies and there’s a whole lot of people keen to see an expedited path to market for these new treatments, IMU is in a good place.
There’s one further consideration working in IMU’s favour as well: Big Pharma is on the hunt for novel combinations of drugs, where traditional treatments are combined with immunotherapy, which can work together to increase patient response rates without the threats of higher toxicity or prohibitive costs...
With Phase 2 set to kick off early next year, IMU could well be on the home stretch to a lucrative deal.
As the small cap’s executive chairperson, Paul Hopper, explained in a recent interview — once a promising clinical asset starts lookin’ the goods in Phase 2, that’s the point when Big Pharma comes sniffing, and offers start flowing in...
On that note let’s hear the latest from:

We wrote about Imugene (ASX:IMU) in Next Biotech only last month, where we covered several major developments from the budding small cap.
At the time, IMU had just announced zero safety issues and it was essentially ‘all systems go’ for its HER-Vaxx trials in Gastric Cancer.
Another significant bit of news was IMU’s acquisition of two new assets through a licencing program with Ohio State University and Mayo Clinic — namely, Key-Vaxx and B-Vaxx — with KEY-Vaxx to be put through clinical trials next year, and B-Vaxx already in Phase 2 at Ohio State University.
You can get a full and in-depth view of the state of the biotech industry and IMU’s role in the interview below in which Paul Hopper broadly discusses the process of ASX biotechs, how they progress drug candidates and clinical trials, and how they typically – everything going to plan – slide into an enormous buy-out sum at the end of the rainbow.
Hopper can speak to the topic with a certain degree of authority considering he led Viralytics (formerly ASX:VLA) to an A$502.4 million buyout from pharmaceutical giant, Merck & Co (NYSE:MRK) earlier this year. Impressively, it was all for Viralytics’ single clinical oncology drug, CAVATAK®.
Discussing the state of the biotech industry in Australia, Hopper states that 10 years ago, there was little foreign investment in Australian biotechs however the landscape today is quite a different story.
Today, the industry is seeing plenty of ‘good exit’ stories like Viralytics. Hopper also referred to the recent acquisition of Sirtex Medical — which was delisted in September post-sale — for $2 billion to Chinese firm CDH Investments. Sirtex's key product is designed to improve the quality of life of patients dying of late stage liver cancer.
Hopper also emphasizes that Phase 2 is ‘where it all happens’ in terms of Big Pharma deals; and considering the huge costs of Phase 2I trials, most smaller biotechs are happy to have a clinical asset picked up for the right price at or just after the second Phase of trials.
Good thing, then, that IMU is headed at full pace for its Phase 2 HER-Vaxx trial in Gastric Cancer.
HER-Vaxx Phase 1b topline results now in
IMU has just released the highly anticipated topline results for its HER-Vaxx trial in Gastric Cancer, and they sound very positive.
The Phase 1b trial involved 14 patients across three dose levels, of which 10 were evaluable for tumour growth assessment during the study.
As mentioned above, of the 10 patients evaluable for tumour growth, 4 showed stable disease (SD), and a further 5 showed partial response (PR) for their best overall response.
To remind you, a partial tumour response means a reduction in the total tumour burden by greater than 30%, and a stable disease response means the total tumour burden either increases less than 20% or decreases less than 30%.
While it’s still early days, these results are encouraging, to say the least.
IMU’s B-cell active immunotherapy concept is gathering positive reinforcement, and providing the team with further confidence as it prepares to kick off the all-important Gastric Cancer HER-Vaxx Phase 2 trials in the New Year.
It’s also a good sign for the small cap’s broader pipeline of B-cell immunotherapies.
There are an estimated 1,300 immune-based treatments now being studied across the world, according to the Cancer Research Institute.
The below graph breaks these down into type and also shows the current clinical stage:
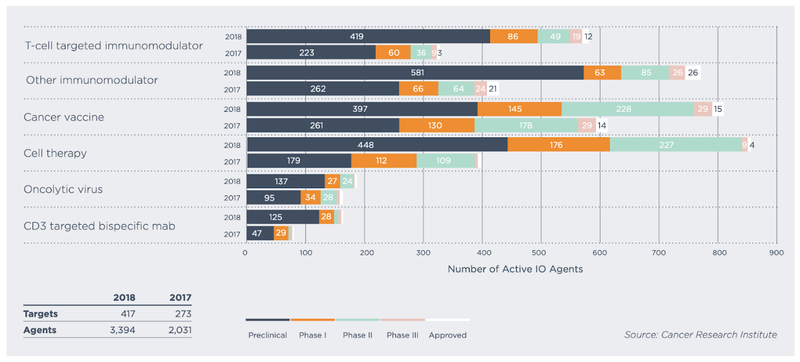
There’s a fair bit more navy blue (treatments at preclinical stage) in there than green (treatments in Phase 2) which gives you an idea of how far IMU’s HER-Vaxx asset has come, and the significance of this milestone.
To reiterate, an increase of antibody levels (immune response) in all patients at all dose levels was observed in the HER-Vaxx trial — which is absolutely key to IMU’s immunotherapy pipeline and platform.
No safety issues were reported, while the drug was also found to be well tolerated.
With these results, the study’s Cohort Review Committee has arrived at a recommended dose for Phase 2 of 50 micrograms.
The Phase 1b study of its HER-Vaxx cancer vaccine targeted patients with advanced stomach cancer expressing the HER2 target protein.
There have been over one million new cases of stomach cancer in 2018 so far, according to the World Cancer Research Fund, and sadly, treatment for stomach cancer is often palliative, because it can be very difficult to cure.
So a new, effective and safe drug in this area would be cause for major celebration. Not to mention, some serious money changing hands.
Looking towards Phase 2...
Now that HER-Vaxx has been green-lit for further trials in Gastric Cancer, the small cap is eager to move onto the next stages as fast as possible.
Phase 2 will test the efficacy, safety and immune response in 68 gastric cancer patients with metastatic gastric cancer over-expressing the HER-2 protein.
It will be randomised into two arms of either HER-Vaxx plus standard-of-care chemotherapy or standard-of-care alone. The primary endpoint is overall survival, with the secondary endpoint being progression-free survival.
In a strategic move by the savvy biotech, the Phase 2 study will be conducted at sites across Asia, Eastern Europe and India where clinicians and patients have difficulty accessing established antibody treatments such as Herceptin and Perjeta marketed by Swiss multinational Roche Holding AG.
The list of countries also comprises several with a particularly high prevalence of gastric cancer. For those playing at home, full study details can also be accessed on clinicaltrials.gov under study ID: NCT02795988.
Skies are looking blue for IMU, with HER-Vaxx and beyond
Novel or improved cancer treatments represent the biggest market out of all biotech markets in the world.
In the last five or so years, an immuno-oncology revolution has taken place, as scientists and researchers look at ways to ‘wake up’ the body to the presence of dangerous cancer cells — in short, to help the body realise that the cancer cells are foreign and therefore need to be killed.
The science, and the approaches to this problem, are becoming more sophisticated by the day.
Small caps with a promising drug candidate in cancer immunotherapies stand to gain huge rewards if they get it right.
In his interview, Hopper drew a picture of chemotherapy as being a bit like throwing weed killer over the lawn. It works, but it also kills a lot of other cells. What immuno-oncology looks to do is achieve a similar outcome (or better) in terms of cancer cell death, but without the same degree of negative effects seen in traditional treatments.
A cancer survivor who is in a better state of health post-treatment will not only have a better quality of life but will be in a better position to fight off a recurrence of the cancer — which for some people, means the difference between life and death.
There is no doubt a land-grab going on in the immuno-oncology space, and ASX biotechs are receiving a lot of interest, with the recent success stories of both Viralytics and Sirtex speaking to this fact.
With IMU now gathering some serious data for HER-Vaxx, and with several other assets in its pipeline, the highly-credentialed and talent-rich small cap is no doubt preparing itself for a big 2019.
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

