IIQ: The mail detecting and destroying cancer now?
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 1,298,000 IIQ Shares and 575,000 IIQ Options and the Company’s staff own 40,000 IIQ Shares at the time of publishing this article. The Company has been engaged by IIQ to share our commentary on the progress of our Investment in IIQ over time. This information is general in nature about a speculative investment and does not constitute personal advice. It does not consider your objectives, financial situation, or needs.
Our biotech Investment Inoviq (ASX:IIQ) is developing an exosome platform technology...
and is looking to apply its exosome platform to develop diagnostics (detecting diseases) AND therapeutics (curing diseases).
An exo-what platform?
Exosomes are tiny, microscopic extra-cellular “containers” that carry ‘messages’ between the human body's cells that allows the human body to function.
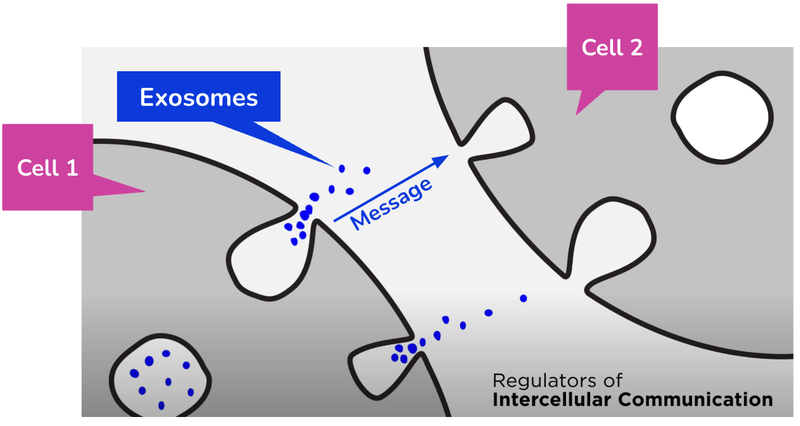
Think of them like postal workers delivering the mail (with important messages) to different houses.
They need to know the location of the house AND the message to deliver to it.
Aside from a healthy body's normal functions, exosomes also carry messages about diseases...
(“don’t get mad, i’m just the messenger”)
By snooping in on what “messages” individual exosomes are carrying, IIQ can detect diseases at a very early stage (diagnostics), often before the body is showing any physical symptoms.
And because the exosome is carrying messages that contain information about the LOCATION of which cells have a disease...
IIQ can insert treatments for that disease into the exosome, which then proceeds to deliver it to the exact cells with the disease.
(a very targeted cellular level attack on a disease compared to the "scorched earth” approach that many treatments use)
Think of it like knowing that a letter is being mailed to the home address of a bad guy...
and inserting a letter bomb.
Wow, our innocent "exosomes are like a mailman” analogy certainly just got pretty dark...
But when it comes to locating and destroying cancer cells before they can spread, that’s fine with us.
Here’s where IIQ is at today and what we are watching for next:
IIQ is going after two types of cancer that have NO approved/recommended treatments or diagnostic tools.
An ovarian cancer screening test AND a treatment for triple negative breast cancer.
(first a very quick summary of both and what we are watching for next. Read on for more detail)
Ovarian cancer screening test.
- Ovarian cancer is usually asymptomatic (no symptoms) in the early stages of the disease. If a woman is diagnosed at stage 1 - the survival rates can be over 90%. If a woman is diagnosed at stages 3/4 survival rates can be ~29%. ~70% of all ovarian cancer in Australia is diagnosed as stage 3 or 4 cancer...
- At the moment there are no recommended screening tests for women who show no symptoms of ovarian cancer (meaning there is no quick and easy screening test that can be regularly taken by all healthy women to catch ovarian cancer early)
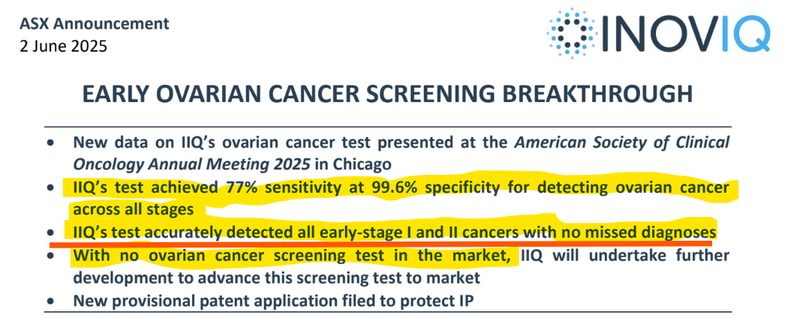
- IIQ’s screening test has been able to detect all stage 1 and 2 ovarian cancers (the earliest but asymptomatic stages where survival rates are the highest)... With over 99.6% specificity (catching 99.6% of all patients with cancer) AND NO misdiagnosis.
- What’s Next: Do a larger clinical validation study (basically seeing if the test works on a larger cohort of patients) then look at “strategic partnering” to commercialise the screening tool.
A therapeutic (cure) for triple negative breast cancer:
- Triple negative breast cancer is an aggressive type of breast cancer that does NOT respond to traditional breast cancer therapies.
- At the moment, there are no approved cell therapies treating triple negative breast cancer.
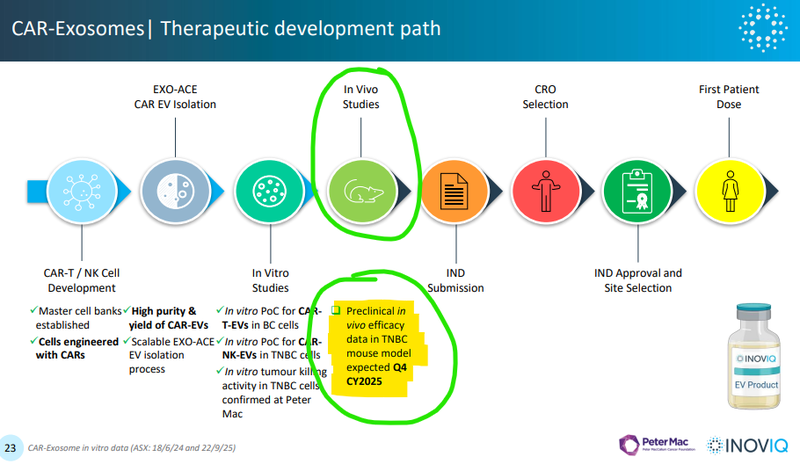
- IIQ is developing the therapeutic under a deal signed with the prestigious Peter MacCallum Cancer Centre (Peter Mac) in Melbourne. The institution is among only a handful globally that combines a dedicated cancer hospital with a fully integrated cancer research program.
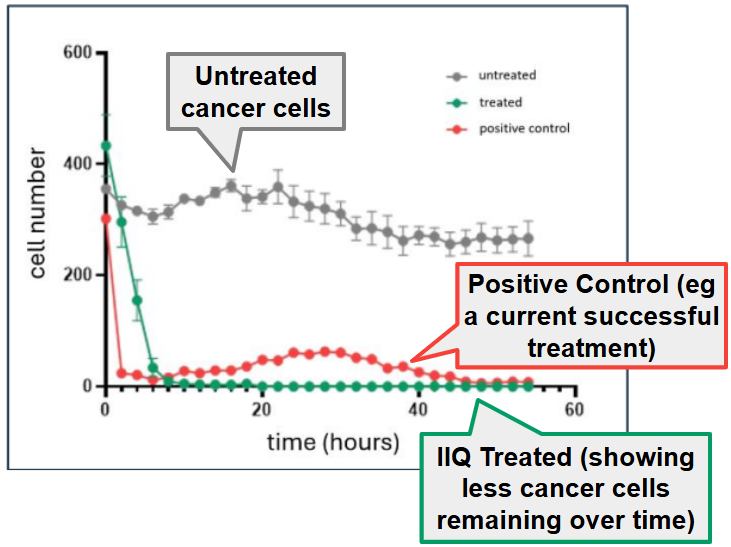
- So far IIQ has shown that its therapeutic was able to kill more than 90% of the cancer cells within 10 hours of treatment in in vitro studies (in a laboratory setting).
- What’s next: Next for the therapeutic is the results from animal studies (in-Vivo) due this quarter.
(Source)
We think that any solid tumour therapeutic IIQ is able to develop could be a game changer for the company.
Mainly because it would de-risk IIQ’s platform tech (if it works for one cancer, why wouldn't it work for others?)...
We originally Invested in IIQ at 50c back in June 2024, and put more cash into the placement last month at 35c.
We think $46.5M capped IIQ finally has a large enough cash runway (~$17.2M in cash after the raise) to deliver major catalysts from its platform tech. (source)
A platform technology (exosomes) that can be applied to three different markets:
- Research Tools (US$661M market size)
- Diagnostic Products (US$6.1BN market size)
- Therapeutics (US$55.3BN market size)

(Source)
The big game changer for IIQ could be therapeutics...
IIQ is developing a therapeutic for triple-negative breast cancer.
At the moment there are no approved cell therapies treating Triple Negative Breast Cancer.
So far IIQ has shown that its therapeutic was able to kill more than 90% of the cancer cells within 10 hours of treatment.
These results were from “in-vitro” (in test tube) studies.
(Source)
This data from IIQ’s cancer therapeutics was so promising that it led to a deal with the prestigious Peter MacCallum Cancer Centre (Peter Mac) in Melbourne.
The institution is among only a handful globally that combines a dedicated cancer hospital with a fully integrated cancer research program.
That means there is a ready and willing cohort of patients to which cutting edge treatments can be quickly deployed in a clinical setting.
Next for the therapeutic is the results from animal models (in-Vivo) due this quarter...
(basically, when we will see how IIQ’s therapeutic performance in animal models)
Success here could put IIQ into the same conversation as all the other cell therapy/exosome companies that were attracting billion-dollar deals...
The last four on the list below were all working on exosome technologies - all did deals at the pre-clinical stage with total deal values ranging from US$882M to US$1.2BN...
(IIQ is currently pre-clinical with its therapeutic)
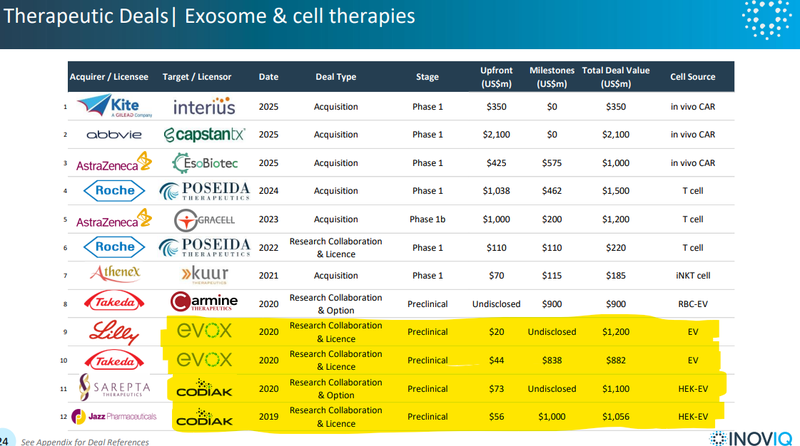
IF IIQ can prove out what could be a more scalable platform tech, then maybe the deal values would be multiples of those in the table above too.
(Source)
IIQ for the first time since we Invested now has a cash runway to deliver major catalysts from its platform tech.
IIQ just raised $9.5M - cornerstoned by Tian An Medicare Limited, who invested $5M into the raise.
Tian An Medicare is a listed company on the Hong Kong exchange that manages and operates hospitals in the APAC region.
It is now a substantial holder of IIQ, holding 10.29% of shares on issue.
IIQ now has ~$17.2M (source) to:
- Progress its ovarian cancer test towards commercialisation (first via a larger clinical validation study), and
- Move its Triple negative breast cancer therapeutic closer to in-human clinical studies (first with animal data).
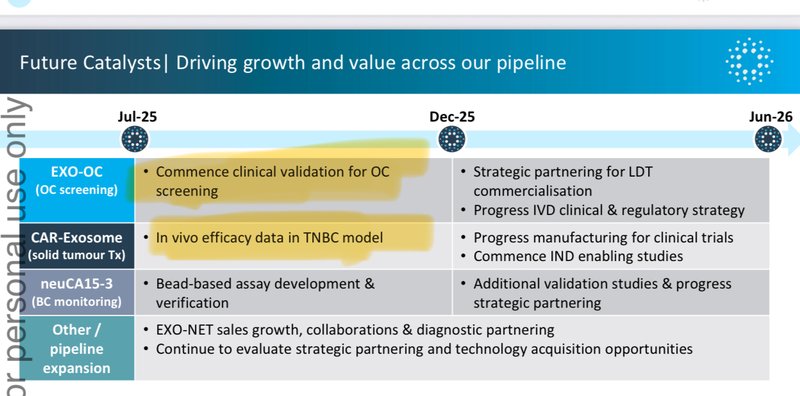
Here are the two main catalysts we are looking forward to seeing from IIQ over the coming months:
In today’s note we will cover in detail:
- Why we like IIQ’s Ovarian Cancer screening tool
- How IIQ can commercialise its Ovarian Cancer screening tool
- How IIQ’s platform tech works
- What we want to see next from IIQ
Why we like IIQ’s Ovarian Cancer screening tool
Ovarian cancer is the world’s deadliest gynaecological cancer.
Ovarian cancer is often called the ‘silent killer’ - as it is usually asymptomatic (no symptoms) in the early stages of disease.
Early detection is incredibly important for ovarian cancer.
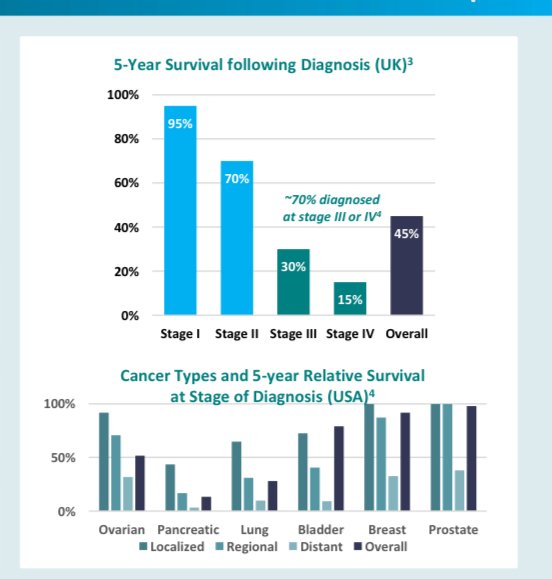
If a woman is diagnosed at Stage 1 - the survival rates can be over 90%.
If a woman is diagnosed at later stages, then the survival rates drop significantly - only 29% of women survive if the cancer is detected at stages 3/4.
The big problem is that ovarian cancer can be asymptomatic in the early stages - meaning most women will never know they had it until it's too late.
For example, ~70% of all ovarian cancer in Australia is diagnosed as stage 3 or 4 cancer...
At the moment there is no way of testing for and detecting ovarian cancer EARLY in women.
IIQ is in a position to develop the first ever test that can be used by all women periodically for a routine check.
Currently, no ovarian cancer screening tests are approved by regulators - due to their poor early-stage detection of cancer.
Earlier this year, IIQ released results that showed its ovarian cancer test was almost 100% accurate across 532 tests.
IIQ’s test accurately detected all early-stage I and II cancer with no missed diagnoses (the earliest stages).
(Source)
Stage 1 and 2 ovarian cancer is usually when patients are asymptomatic...
At the moment ~70% of ovarian cancer is diagnosed in stages 3 and 4.
Those stages are also the periods where early detection can mean the highest survival rates:
With IIQ’s recent “breakthrough” results, the company has the opportunity to develop and commercialise the first-ever “population screening” tool for ovarian cancer.
(this means anyone can quickly and easily take the screening test)
Now IIQ is focused on getting enough data to achieve regulatory approval for its ovarian cancer screening test, before it can be commercialised.
The world needs an early stage ovarian cancer test that can be used by all women (not just women who are already known to be high risk), and IIQ may have demonstrated they can develop it...
The big opportunity now for IIQ is for its screening test to be used by all healthy women periodically for a routine ovarian cancer check.
Similar to the way men are routinely tested for prostate cancer...
There is currently US$323M spent each year on screening for ovarian cancer - and that's just in high risk patients...
To be able to detect ovarian cancer early, all women need to be tested, not just high risk women, which could make the screening market for ovarian cancer a lot bigger than it is today.
Ovarian cancer affects over 1 million people each year, and is the world’s deadliest gynaecological cancer.
According to the following slide from IIQ’s presentation a screening tool could potentially reach ~243M women every 1-2 years only across the 9 major markets globally:
(that is a lot of screening tests...)
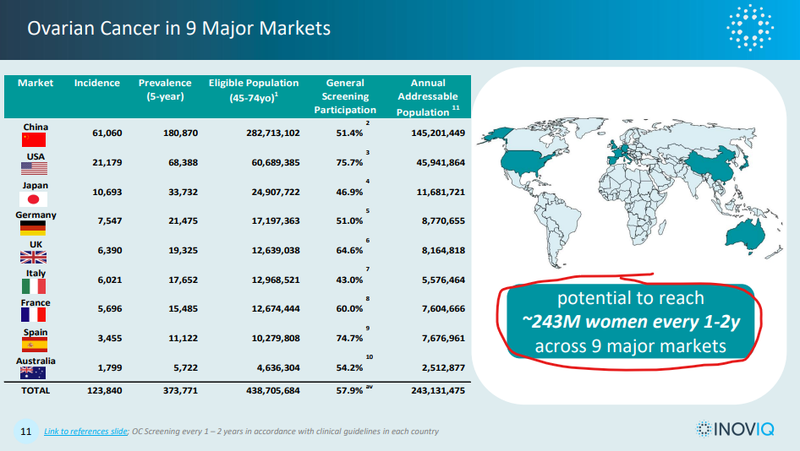
(source)
IIQ’s pathway to regulatory approval for its ovarian cancer screening test
There are two paths that IIQ can pursue to get regulatory approval for its screening test:
- Lab Developed Test (small commercialisation pathway, faster to get real world data)
- In Vitro Diagnostic pathway (standard pathway for FDA approval of medical tests)
(more on both those pathways below)
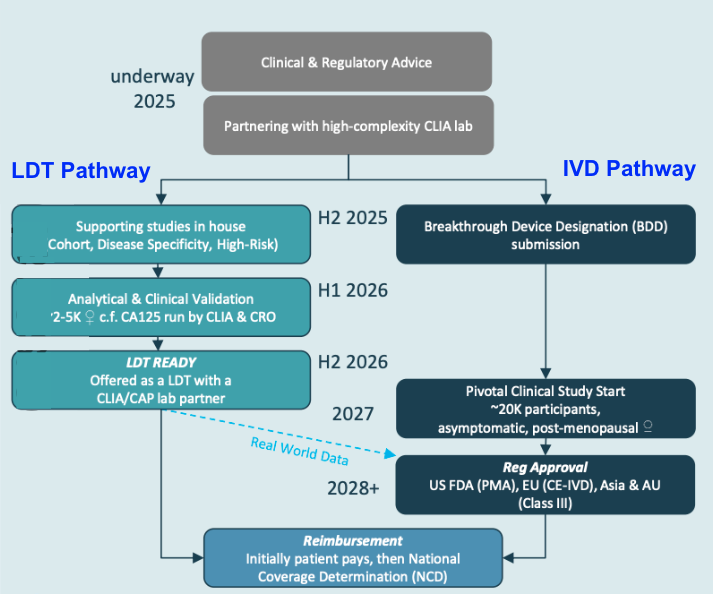
(Source)
Lab Developed Test (LDT) pathway to commercialisation
A Laboratory Developed Test (LDT) is a medical test that’s designed, created, and used inside a single certified lab, rather than being sold as a commercial test kit.
IIQ will be looking for a partner (a physical lab) in the United States to develop a Laboratory Developed Test (LDT).
This will be a central place where patients can come in and undertake IIQ’s test - granting IIQ very valuable “real world data” to advance its regulatory approval.
The LDT is a faster way for IIQ to get its test patients (in a lab setting rather than an off the shelf test) and gather some real world data in anticipation for a larger study.
A company developing an ovarian cancer screening test might choose the LDT pathway before, clinical validation study to:
- Prove demand for its product
- Collect more patient data and potentially optimise the algorithm
- Attract partners or investors for a larger clinical validation study.
In Vitro Diagnostics (IVD) pathway to commercialisation
The IVD pathway is the FDA’s formal process for proving test works and then getting permission to sell it as a medical device.
IIQ will first seek “Breakthrough Device Designation”, which will be the first hurdle under this pathway.
Breakthrough Device Designation is a special fast-track pathway for medical devices and diagnostics that could dramatically improve patient care.
Given IIQ’s results so far (where there were no misdiagnoses), we think there is a chance that IIQ could achieve this milestone.
With Breakthrough Device Designation, IIQ will then undertake a much larger clinical validation study (roughly 20,000 patients).
Think of this like a Phase 3 study before regulatory approvals.
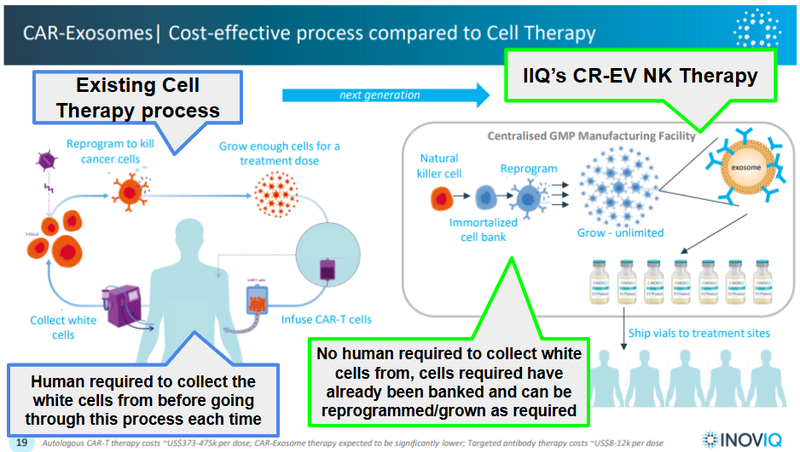
How does IIQ’s technology work?
IIQ has a proprietary exosome platform with multiple research, diagnostic, and therapeutic applications.
Exosomes are tiny “packages” released by all human cells to help them communicate with each other.
Exosomes are 200 times smaller than a cell.
They work by carrying molecular cargo (like DNA and proteins) that act as cell messages.
These messages can also be used as biomarkers for disease and help humans understand and diagnose whether someone has a particular disease.
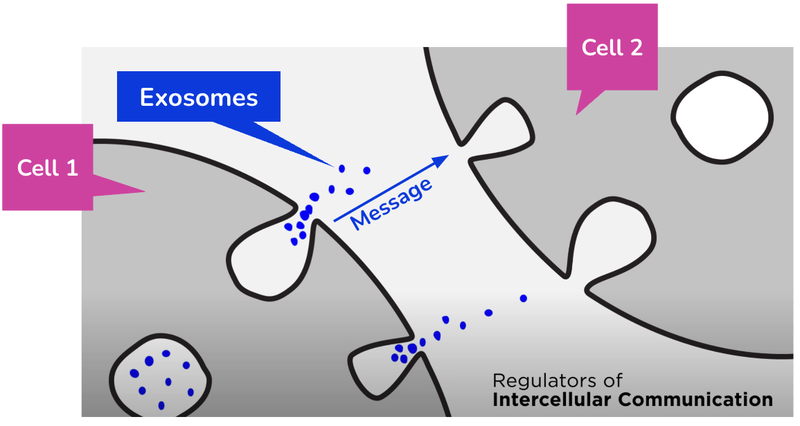
Think of exosomes as the “couriers” for messages being delivered around the human body.
They pick up and drop off messages (biological information) to different parts of the body which trigger tailored responses from different cells.
These messages have information, and if IIQ is able to identify what that information is, it is able to detect issues in the body like cancer.
EXO-NET is IIQ’s proprietary technology to capture these incredibly tiny molecules.
Through IIQ’s EXO-NET product (which IIQ is generating revenue from right now selling to universities and other companies studying the effects of exosomes), IIQ is able to develop other exosome based-products.
The first one we have covered well today, the ovarian cancer screening test, the second one is cancer therapeutics for cancer tumours.
What is next for IIQ?
Here is a quick summary of the key catalysts we will be watching out for over the coming months:
Ovarian Cancer Test
- 🔄 Secure a partner / physical lab for clinical validation studies.
- 🔄 Secure breakthrough device designation
Solid Tumour Therapy
- 🔄 Results from solid tumour therapy in animals
Other
- 🔄 Development of exosome diagnostic for neurodegenerative conditions like Alzheimer’s and Parkinson's. (Diagnostic development to start this quarter)
What could go wrong?
Although the data published by IIQ is extremely promising, there is still a risk that the technology fails in a clinical validation study.
The technology is still at an early stage, and while IIQ has limited revenue streams, there are always risks with emerging technologies.
Technology risk
Exosomes are still an emerging field of scientific research, and there may be undiscovered drawbacks to exosomal products, in particular as they apply to therapeutics.
For example, IIQ’s preclinical research on exosome therapies may not yield the desired results or may not be deemed acceptable in the eyes of a regulatory body.
Source: “What could go wrong - IIQ Investment Memo 2024”
To see more risks, check out our IIQ Investment Memo here.
Other risks
Like any tech healthcare small-cap investment, investing in IIQ carries a range of risks that may affect the value of the company, including risks that may not be foreseeable (this is the nature of risks).
Here we aim to identify a few more risks.
The company’s progress is dependent on external market conditions. A downturn in biotech, diagnostics, or broader risk-asset markets could impact IIQ’s ability to attract partners, secure non-dilutive funding, or raise capital on favourable terms.
As a pre-revenue small cap, IIQ relies heavily on capital markets to fund ongoing R&D and commercialisation. Any future capital raising may dilute existing shareholders, especially if conducted during weaker market conditions or after slower-than-expected progress.
Operational and execution risks remain. Scaling a novel diagnostics or therapeutic platform involves complex manufacturing, quality control, regulatory compliance, and supply chain requirements. Delays or technical setbacks could affect development timelines.
Competitive pressure is another factor. The diagnostics and precision-medicine sectors are crowded and fast-moving; competing platforms or larger incumbents may develop superior performance, speed to market, or regulatory positioning.
Partnership and commercialisation risks also exist. There is no guarantee that discussions with potential partners lead to binding agreements, or that partners deliver the level of investment, data access, or market reach the company anticipates.
Finally, given IIQ’s early stage, the current share price may already reflect expected future milestones, which increases the risk of sharp volatility if updates do not meet market expectations.
Investors should carefully consider these risks and seek professional advice tailored to their personal circumstances before investing.
Our IIQ Investment Memo
Our Investment Memo provides a short, high-level summary of our reasons for Investing. We use this memo to track the progress of all our Investments over time.
In our IIQ Investment Memo, you can find the following:
- What does IIQ do?
- The macro theme for IIQ
- Our IIQ Big Bet
- What we want to see IIQ achieve
- Why we are Invested in IIQ
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

