IIQ: Exosomes successfully weaponised - cancer cells located and killed in proof of concept study
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 1,150,000 IIQ Shares and 525,000 IIQ Options at the time of publishing this article. The Company has been engaged by IIQ to share our commentary on the progress of our Investment in IIQ over time.
Cancer cells directly targeted...
located...
and killed.
...in the lab.
Tiny extra cellular material was armed with cancer killing abilities...
and it worked.
A big, early step on the pathway to developing a cancer therapeutic for humans.
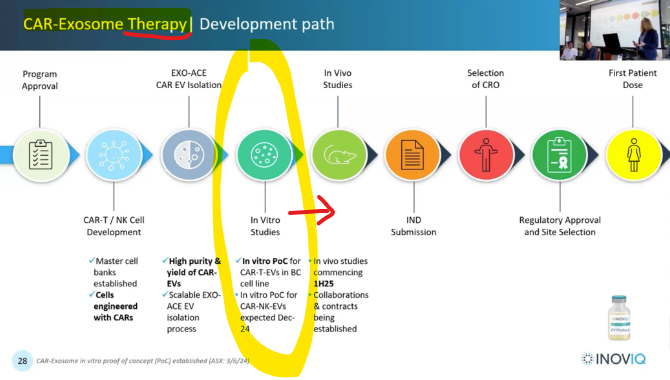
Today Inoviq (ASX:IIQ) confirmed it has successfully completed stage 1 of its development program for an exosome therapeutic for breast cancer.
IIQ was able to engineer cells to continuously produce exosomes that specifically target and kill breast cancer cells.
After today’s Proof of Concept study, IIQ says to expect more valuable lab data to come in the first half of 2025.
... then IIQ will move onto mice models in the second half of 2025.
A prelude to eventual human trials at some point in the future.
Previously we have written a lot about IIQ’s diagnostic capabilities.
Diagnostics is DETECTING early stage cancer.
If you can detect cancer early, you are in a much better position to treat it.
However IIQ is also developing therapeutic technologies - to KILL cancer cells.
This means IIQ is moving more into the higher value $66BN therapeutics market.
Just like in its diagnostics work, IIQ is leveraging the use of exosomes in its therapeutic platforms - exosomes are extra-cellular “containers” released by ALL human cells.
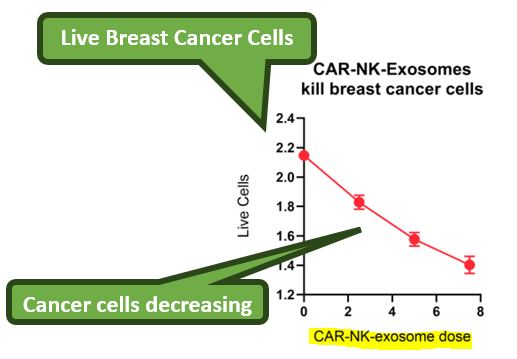
Exosomes can carry messages - and increasingly it looks like they can also be successfully ‘weaponised’ - which is how IIQ was able to locate and kill cancer cells in the lab.
In the Proof of Concept study released today, IIQ was able to kill 30% of triple negative breast cancer cells (a particularly aggressive type of cancer) in a test tube.
IIQ did this by attaching something called a chimeric antigen receptor (CAR) to natural killer (NK) cells which are part of the body's immune system.
Think of a CAR as a targeting device that helps the body's immune system find and kill cancer cells.
Regular readers may remember that one of our other Investments, Arovella Therapeutics (ASX: ALA) is also developing cancer therapeutics with CARs and invariant natural killer (iNKT) cells for solid tumours.
ALA has been one of our best performing Investments of the last couple years and is currently up 334% from our Initial Entry.
In terms of where the two companies are at - ALA is pre-clinical, having already done animal studies.
ALA is currently capped at $174M.
It just goes to show just how much the market values even early stage pre-clinical advancements in cancer therapeutics.
IIQ is looking to go into animal studies for its cancer cell treatment in H2 2025.
IIQ is capped at $58M.
With the prospect of IIQ growing into a company that has BOTH extremely accurate cancer diagnostics AND highly promising early stage cancer therapeutics, we are looking forward to a busy year of newsflow for the company.
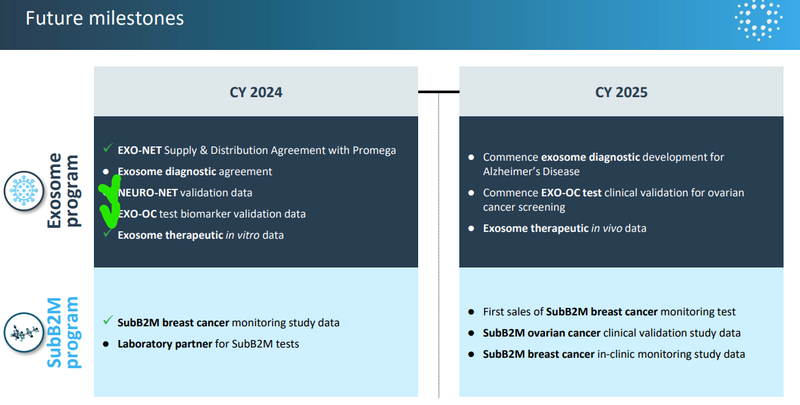
In particular we are looking forward to the following catalysts:
- 🔄Updates to come based on today’s progress: a further in vitro (test tube) study then in vivo (mice study) data on solid tumour therapy
- 🔄A lab partner for SubB2M tests (for breast cancer monitoring)
- 🔄Clinical validation studies for highly accurate ovarian cancer screening test
- 🔄Development of exosome diagnostic for neurodegenerative conditions like Alzheimer’s and Parkinson's.
We think its a great mix of commercial and research catalysts across a range of large markets, which gives IIQ an excellent set of opportunities to deliver a large, long-term re-rate.
Reminder: What has IIQ achieved since we Invested?
Below are our two recent notes which cover what IIQ has so far delivered this year, and what we can look forward to next year:
A potential breakthrough in ovarian cancer screening:
Read: IIQ announces extremely accurate cancer screening test - 94%
Biomarker validation that may help with the early detection of Parkinson's and Alzheimer's:
By way of reminder we’ve also got the key reasons we Invested in IIQ below as well:
The 9 key reasons we Invested in IIQ
Below are our 9 key reasons for Investing in IIQ. This is an excerpt from our May 2024 initiation note on the company, along with any material updates since then in italics.
- Going after big markets with potential breakthrough technology - IIQ is developing a technology that will detect cancer and possibly neuro diseases like Alzheimer’s. These are big markets and its platform technology could capture the interest of Big Pharma if it is able to prove that it works in a clinical setting.
- Strong board - the team from success story Polynovo - IIQ has three directors who are all with or previously with Polynovo, one of the great ASX biotech success stories of the last decade. Polynovo re-rated from ~4c to ~$4 in the space of 4 years and it remains capped at ~$1.7BN at the time of this memo.
- Merchant Funds Management are the biggest shareholder - Merchant is IIQ’s biggest shareholder holding ~14% of the company. Merchant has had a lot of success investing in Biotechs including - Race Oncology which went up ~2,900% (low to high) and the IIQ’s board other company Polynovo, up ~10,025% at its highest point.
- Platform technology to deliver multiple “shots on goal” - IIQ is somewhat different to a lot of biotechs out there in that its exosome technology platform doesn’t limit it to a binary outcome on a single clinical trial, if one catalyst doesn’t work out, there are more to look forward to.
- Tight cap structure - With just ~100M shares on issue and Merchant holding ~14% and Chairman David Williams holding ~5% there aren’t many shares floating around. This generally means that with fewer shares in the market, it’s more likely to re-rate on major news.
UPDATE: IIQ’s capital structure still remains extremely tight. The company now has ~ 111.5M shares on issue. - IIQ’s breast cancer test is more accurate than leading test - A recent clinical validation study of IIQ’s breast cancer test showed that it detected 19% more breast cancers than the leading test (CA15-3 test by $332BN Roche) with an impressive 81% sensitivity and 93% specificity across a range of breast cancers.
- Early stage moonshot to treat solid tumours - IIQ is also advancing a solid tumour therapy which has been shown to kill 75% of breast cancer cells in 72hrs in an in vitro (test tube) study. There is a huge unmet need for treatments in the solid tumour space where, so far, cell therapies haven’t been developed.
NEW: Today’s news re-affirmed this reason & now IIQ is looking to move its therapeutics product into animal studies next year. - Expansion of tests to include ovarian cancer and neurodegenerative diseases (Alzheimer’s) - IIQ is looking to apply its exosome platform technology to ovarian cancer, and more recently has been working on neurodegenerative diseases such as Alzheimer’s. A blood test for the early detection of Alzheimer’s would be a major scientific advancement.
- Large commercial opportunities from underlying technology - IIQ has an exosome isolation product which is already being sold to research groups. IIQ also has commercial agreements with Promega and a European biotech to bring IIQ’s exosome platform technology to market. Because IIQ’s exosome technologies can be applied to diagnosing and potentially treating many different diseases, we expect to see more partnerships where IIQ licences their exosome tech.
Check out our full IIQ Investment Memo to see the key objectives we want to see IIIQ achieve, risks we have identified and accepted and our Investment plan.
Ultimately, we hope that a combination of the above reasons contribute to IIQ achieving our Big Bet, which is as follows:
Our Big Bet for IIQ
“IIQ re-rates to a +$500M market cap on commercialisation of its breast cancer test, its ovarian cancer test, neurodegenerative disease test and/or its solid tumour (breast cancer) therapy.”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our IIQ Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
Weaponised exosome update from AGM - key points
Click here to listen to the section of the recent IIQ AGM where the IIQ CEO talks about the applications for therapeutics (actually treating/killing cancer).
~15 minutes into the presentation CEO Dr Leearne Hinch starts talking about “weaponised exosomes”:
(Source)
What is IIQ’s exosome technology?
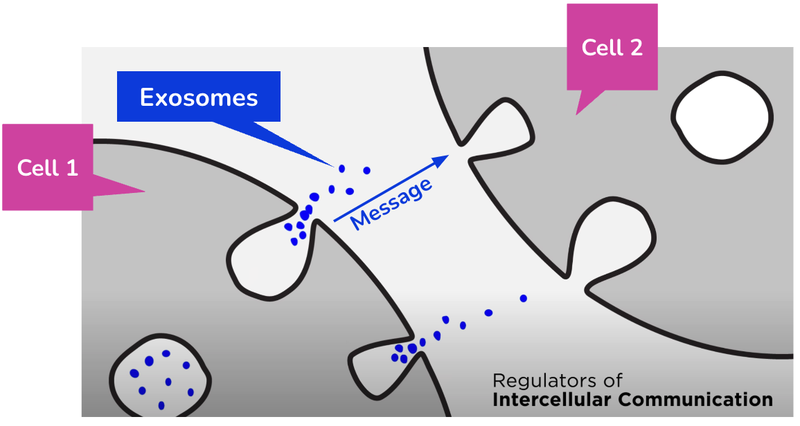
Exosomes are naturally occurring delivery messengers between cells.
Below is a concise video which summarises what exosomes are and how they work:

(Source)
Think of exosomes as the postal service in the human body - they deliver packages with precise instructions and information, helping cells communicate.
As a result, they can also act as a sort of cellular Border Force - finding out where the bad guys are (cancer and other diseases) and what they are up to.
But because they travel around so much and can move between cells - it is believed that they can also deliver powerful therapies that can kill bad guys (cancer and other diseases) as well.
A good guy that has access to the bad guy's conversations - and can also bring the bad guy to justice.
Control the “messenger,” find out what’s happening in the cell AND (hopefully) get the messenger to deliver potentially life saving therapies.
That’s what IIQ is showing its technology is capable of doing.
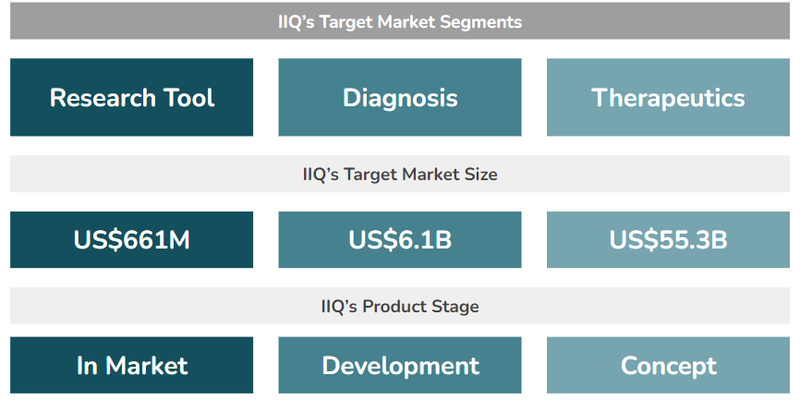
IIQ is looking to take its exosome technologies and apply it to three different markets:
- Research Tools (US$661M market size) - IIQ has an exosome-based tool used by researchers to enable biomarker discovery (identifying diseases) and validation (supporting the research of new treatments). This tool is already generating revenues for the company.
- Diagnostic Products (US$6.1BN market size) - IIQ is developing exosome-based tests for screening and monitoring for various conditions, including breast cancer, ovarian cancer and neurodegenerative diseases like Alzheimer’s.
- Therapeutics (US$55.3BN market size) - A “weaponised” exosome engineered to target and treat solid tumours. Today’s news relates to this market.
What’s next for IIQ?
So far IIQ has published all of the clinical data that it had in the pipeline without delay, which is very impressive for an early stage biotech company.
Also of note is that IIQ is still looking for commercial arrangements for its exosome based tests.
These are generally research organisations that could provide immediate revenue for the company.
However, we think the bigger play is still around the diagnostics tools that IIQ is developing as well as the “moonshot” cancer therapy that will hopefully be advanced more next year...
There was great progress on that today, but we are still mindful of the following risks.
What could go wrong?
The main risk relevant to today’s announcement from IIQ is “technology risk”.
The data published by IIQ today was all “in vitro” meaning it was based on trials done in the lab (test tubes).
IIQ expects to be taking its therapeutic product “in vivo” (animal studies) in H2-2025.
There is always a risk that once the therapeutic goes into animal studies, it isn't as effective or doesn't work at all.
If the tech isn't able to replicate the same (or better) results in animal studies then it could re-rate the IIQ share price lower (especially if the share price runs off the back of today’s news).
To see more risks, check out our IIQ Investment Memo here.
Our IIQ Investment Memo
Our Memo provides a short, high-level summary of our reasons for Investing. We use this memo to track the progress of all our Investments over time.
In our IIQ Investment Memo, you can find the following:
- What does IIQ do?
- The macro theme for IIQ
- Our IIQ Big Bet
- What we want to see IIQ achieve
- Why we are Invested in IIQ
- The key risks to our Investment Thesis
- Our Investment Plan
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

