G Medical continues expansion, receiving TGA approval for GMP
Published 05-MAY-2020 16:02 P.M.
|
3 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Shares in medical device and telehealth company G Medical Innovations Holdings Ltd (ASX:GMV) surged more than 20% on Tuesday morning after the company announced that it had been granted Therapeutics Goods Administration (TGA) approval for its G Medical Extended Holter Patch (GMP).
This marks a significant milestone for the company as the GMP now officially complies with all relevant Australian medical and safety requirements as a Class IIa medical device.
TGA certification adds to the previously granted European ‘CE’ Class II approval received for the GMP.
The G Medical Patch (GMP) is part of the modular Vital Signs Monitoring System (VSMS), an easy-to-use clinical-grade solution for monitoring patients throughout the healthcare lifecycle.
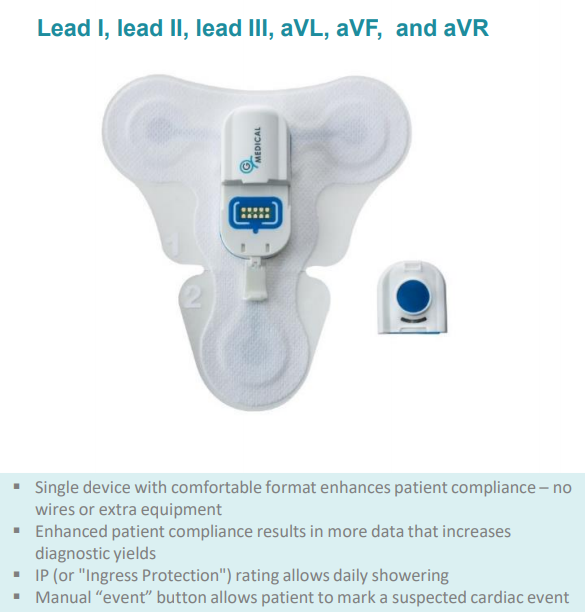
Ideal for use in clinics, assisted living residences, hospitals and with healthcare providers, the GMP streamlines and simplifies healthcare and patient monitoring by delivering continuous, 14 day recording of six-channel ECG.
Importantly, G Medical is also pursuing required regulatory approvals for its GMP in other target markets, including the US.
The GMP seeks to streamline patient monitoring across all stages of the healthcare lifecycle, including pre-hospitalisation, hospitalisation and post-discharge.
The product allows for a higher level of patient care and takes the burden off medical personnel, leading to increased efficiency, decreased liability and a reduced number of re-admissions.
Discussing the significance of this development, as well as pointing to the prospects of other devices developed and being distributed by the group, chief executive Dr Yacov Geva said, “TGA certification is a significant milestone for G Medical and demonstrates how the company’s suite of medical devices and telehealth technologies are relevant to the current environment.
“The company continues to pursue regulatory approvals for both the GMP and the Prizma device in its target markets and looks forward to updating shareholders as they are granted.”
As indicated below, the company has already had significant global success with its Prizma device, including over-the-counter approval by the FDA the US.
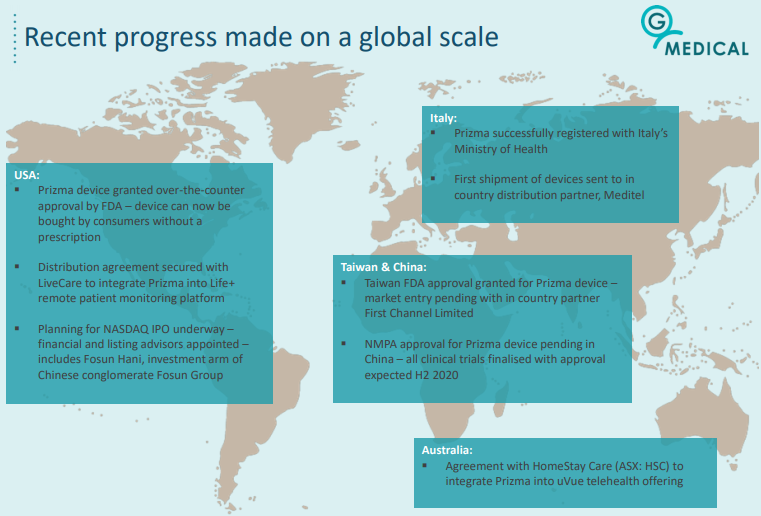
Generating diversified recurring revenues
G Medical specialises in innovative next-generation mobile and e-health solutions using its suite of proprietary devices and software solutions, as well as patient service operations, with a view to driving multiple and recurring revenue streams, across numerous verticals and territories.
The group’s medical devices and telehealth platforms are becoming recognised as cost effective and scalable solutions to ease the burden on healthcare systems, which can be adopted and utilised by both the patient and their physicians with relative ease.
The products are not only effective and relevant in the current COVID-19 pandemic, but are also important for the monitoring and remote management of patients with chronic diseases as well as other specific medical indications, in parallel and beyond COVID-19.
The company has had rapid success across a broad range of markets with its Prizma device which allows consumers to turn their smartphone into a mobile medical monitor to measure a wide range of vital signs, with the added advantage that users are able to store their medical data in the cloud and share it with third parties such as healthcare professionals and family members.
G Medical also offers a professional real-time patient continuous monitoring solution, G Medical’s Vital Signs Monitoring System (VSMS) and G Medical Patch (GMP).
This modular solution measures a wide range of vital signs that are automatically presented in a call centre (IDTF) or a hospital setting.
The GMP assists in diagnosing patient complaints and conditions remotely, from pre-hospitalisation, hospitalisation, and through to post-discharge home-based settings.
General Information Only
This material has been prepared by StocksDigital. StocksDigital is an authorised representative (CAR 000433913) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

